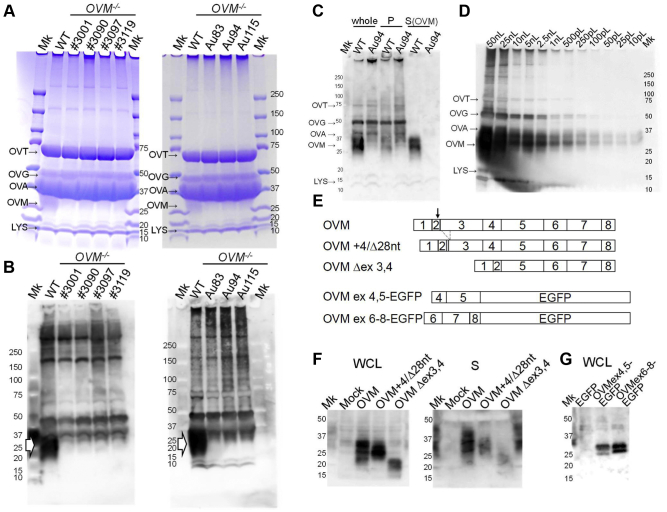
Figure 4.
Reduction of OVM protein in OVM-null egg. (A) SDS-PAGE of WT and OVM-null egg whites stained with Coomassie Brilliant Blue. Each lane is labeled with OVM−/- hen IDs. Major egg white components are indicated. OVT, ovotransferrin; OVG, ovoglobulin; OVA, ovalbumin; OVM, ovomucoid; LYS, lysozyme. Per lane, 500 nL of egg white sample was loaded. (B) Immunoblot analysis of WT and OVM-null egg white proteins with anti-OVM antibodies. The arrowheads indicate the position of OVM protein recognized by anti-OVM antibodies. Each lane contained 50 nL of egg white samples. (C) Immunoblots of partially purified WT and OVM-null egg white proteins, using anti-OVM antibodies. Following acid and heat treatment, WT and OVM-null egg white (derived from Au94 OVM−/− hen) were centrifuged to separate precipitate (P) and supernatant (S). Heat-stable OVM protein was prominent in the supernatant and other egg white proteins accumulated in the precipitate. Each lane contained 10 nL of whole egg white samples. (D) Sensitivity tests of anti-OVM antibodies. Each lane (labeled at top) was loaded with different amounts of WT egg white. Immunoblotting shows that anti-OVM antibodies can detect OVM in 10 pL of egg white. (E) Schematic diagram of proteins expressed in cultured cells. Numbers indicate OVM exons. The gray box and dotted lines, respectively, indicate a four-nucleotide insertion and 28-nucleotide deletion in the OVM+4/D28 nt mutant. Arrow shows cleavage site in OVM. (F) Immunoblots of recombinant OVM mutants with anti-OVM antibodies. Samples were whole cell lysate (WCL) and culture S from HEK293 T cells transfected with WT and mutant OVM. Mock: mock transfection with empty plasmid pcDNA3. (G) Immunoblot of the independent OVM region. Whole cell lysate from HEK293 T cells expressing enhanced green fluorescent protein (EGFP) fused with OVM protein (exons 4–5 and exons 6–8) were subjected to immunoblotting using anti-OVM antibodies.