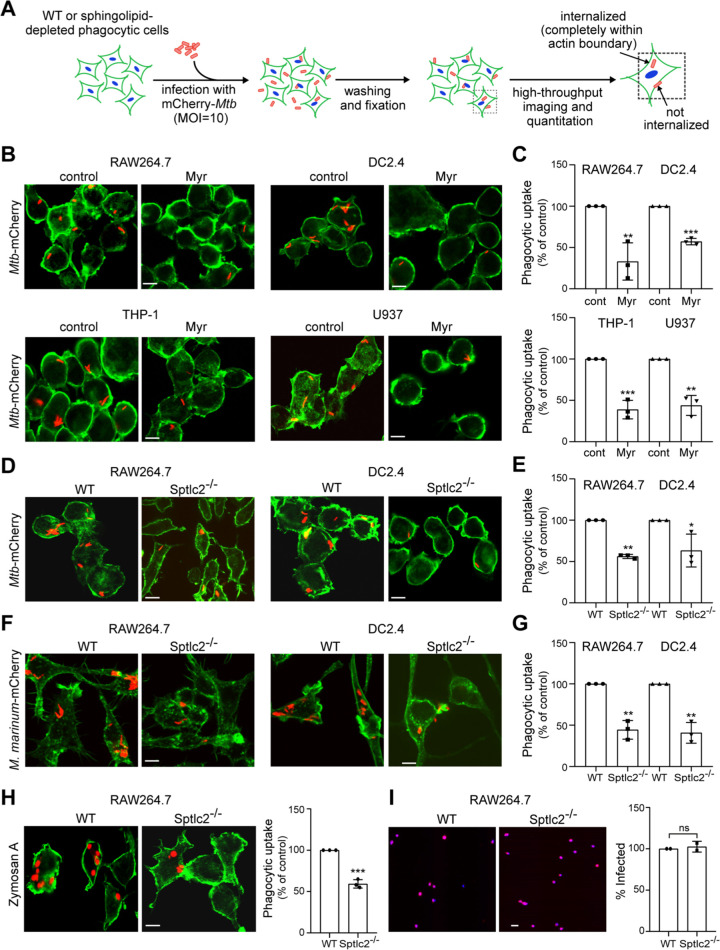
FIG 2.
Sphingolipid biosynthesis is required for efficient phagocytosis of Mtb. (A) Control and sphingolipid-depleted phagocytic cells were infected with mCherry-expressing Mtb at an MOI of 10 for 2 h, washed, fixed, stained with phalloidin Alexa Fluor 488 and DAPI, and then analyzed by high-throughput fluorescence imaging. The efficiency of phagocytic uptake was determined by dividing the total number of internalized bacteria by the total number of cells. (B) RAW264.7, DC2.4, THP-1, and U937 cells were grown in the presence or absence of myriocin for 3 days, infected with mCherry-expressing Mtb, and then processed as for panel A. (C) Quantification of Mtb uptake by cells treated as for panel B. (D) Wild-type and Sptlc2−/− RAW264.7 and DC2.4 cells were infected with mCherry-expressing Mtb and then processed as for panel A. (E) Quantification of Mtb uptake by cells treated as for panel D. (F) Wild-type and Sptlc2−/− RAW264.7 and DC2.4 cells were infected with mCherry-expressing M. marinum at an MOI of 10 for 2 h and then processed as for panel A. (G) Quantification of M. marinum uptake by cells treated as for panel F. (H) Wild-type and Sptlc2−/− RAW264.7 cells were incubated with zymosan A particles at an MOI of 1 for 1 h and then processed as for panel A. (I) Representative images of wild-type and Sptlc2−/− RAW264.7 cells infected with mCherry-tagged herpes simplex virus (HSV) at an MOI of 1. At 12 h postinfection, cells were fixed, stained with DAPI, and analyzed by high-throughput fluorescence microscopy to determine the percent infected cells. Bar, 5 μm. Data in panels C, E, and G are means ± SD (n = 3). *, P < 0.05; **, P < 0.01, ***, P < 0.001 (unpaired t test). Data in panel I are means ± standard errors (SE) (n = 2). ns, not significant by two-tailed unpaired t test.