FIG 6.
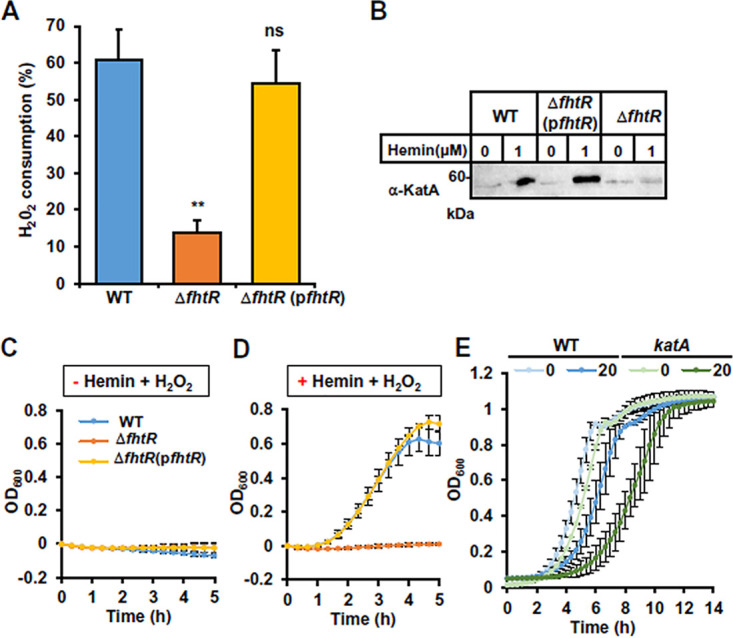
FhtR controls intracellular utilization of heme. (A) fhtR deletion limits heme-dependent KatA activity. KatA enzymatic activity in E. faecalis was assessed on WT, ΔfhtR, and ΔfhtR(pfhtR) grown overnight (ON) with 1 μM hemin. Catalase activity was determined on an equivalent number of bacteria incubated with 100 μM H2O2 for 1 h with the spectrophotometric FOX1 method based on ferrous oxidation in xylenol orange. Results are expressed as the percentage of H2O2 metabolized in respective strains grown without hemin. Results represent the means plus standard deviations from three biological replicates. Statistical significance was determined by one-way analysis of variance (ANOVA) with Dunnett’s multiple-comparison test comparing each strain to the WT strain control with significance indicated as follows: ns, P > 0.05; **, P = 0.008. (B) Expression of KatA is impaired in the ΔfhtR mutant. Equivalent amounts of protein (20 μg) from lysates of WT, ΔfhtR, and ΔfhtR(pfhtR) strains as described for panel A were separated by SDS-PAGE, and immunoblots were probed with an anti-KatA polyclonal antibody. The presented results are representative of three independent experiments. (C and D) The ΔfhtR mutant is hypersensitive to H2O2. ON cultures of WT, ΔfhtR, and ΔfhtR(pfhtR) strains were diluted to an OD600 of 0.01 and grown to an OD600 of 0.5 in the absence of hemin (C) or in the presence of 1 μM hemin (D). Cultures were distributed in wells on a 96-well plate and supplemented with 2.5 mM H2O2 or not supplemented with H2O2. OD600 was monitored every 20 min in a microplate spectrophotometer (Spark; Tecan). OD600 at time zero was normalized to 0. Results represent the means ± standard deviations from three biological replicates. (E) KatA limits heme toxicity. ON cultures of WT and katA::tetR strains were diluted to an OD600 of 0.01 and grown in 96-well plate in the presence of the indicated concentration of hemin as described for panel D. Results represent the means ± standard deviations from three biological replicates.
