Figure 2. Dynamics of the thioester-intact Cpa polyprotein under force.
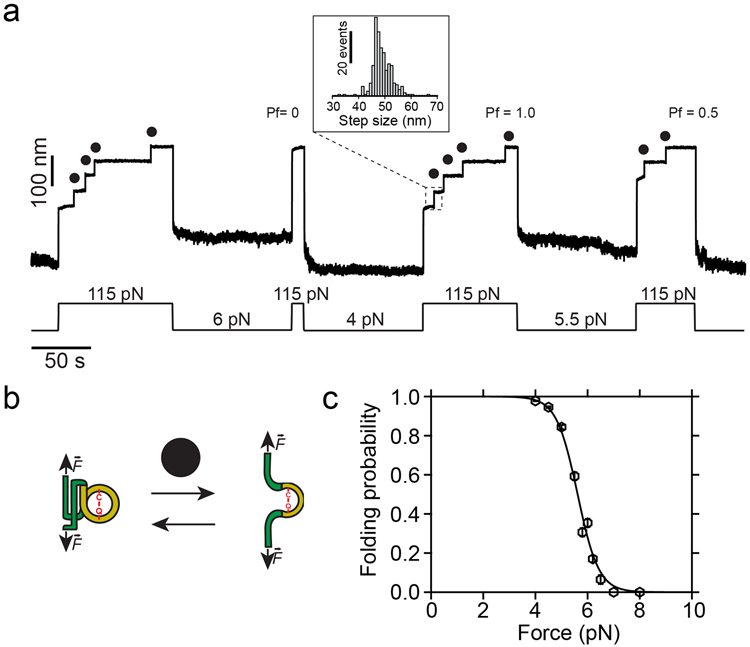
a) Magnetic tweezers trajectory of the Cpa polyprotein. High force pulses at 115 pN unfold the thioester-intact Cpa domains, which show 48.8 ± 3.8 nm (mean±SD, n=272) stepwise extensions (inset histogram). Low force pulses of 100 seconds long allow Cpa refolding, enabling us to determine the folding probability (Pf) at different forces. As an example, a quench at 6 pN does not allow folding of any of the domains, while the four-fold at 4 pN (Pf=1.0), and only two-fold at 5.5 pN (Pf=0.5). b) Cartoon representation of the folding-unfolding of the Cpa domain. The thioester bond between Cys426 and Gln575 clamps the TED domain (yellow), limiting its extensibility. c) Folding probability of thioester-intact Cpa. Data points are fitted to a sigmoidal function and they represent the probability at each of the forces tested for 100 s (n=54 at 4 pN; n=30 at 4.5 pN; n=18 at 5 pN; n=16 at 5.5 pN; n=16 at 5.8 pN; n=23 at 6 pN; n=14 at 6.2 pN; n=10 at 6.5 pN; n=9 at 7 pN; n=5 at 8 pN). Data points are the mean and the bars are the SD calculated using a jackknife analysis.
