Figure 4. Protein folding drives thioester bond reformation.
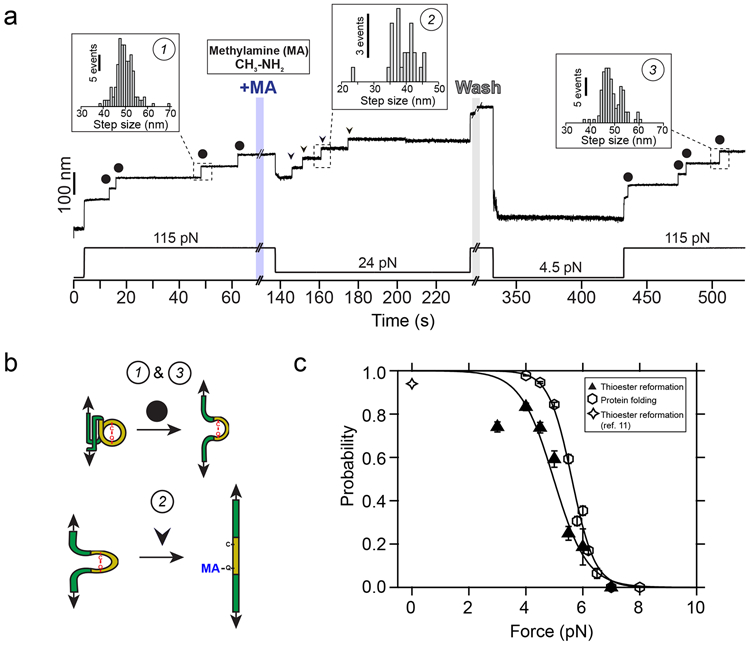
a) Magnetic tweezers trajectory of the Cpa polyprotein. After the unfolding of the thioester-intact Cpa domains at 115 pN (circles; inset histogram #1: 49.6 ± 4.1 nm, mean±SD, n=164), the buffer is exchanged and the polyprotein is exposed to a solution containing 100 mM methylamine (+MA). As expected, we do not observe cleavage at this high force, but a drop to 24 pN permits the full cleavage of the four candidate thioester bonds (arrows, inset histogram #2; 38.8 ± 4.4 nm for 24 pN, mean±SD, n=25). To study the reformation of the bond, we remove the nucleophile-containing buffer at high force, and quench the force to 4.5 pN for 100 s to favor bond reformation and protein folding. We stretch again the polyprotein at 115 pN and identify four thioester-intact Cpa domains, which indicates that the four cleaved candidates were able to fold and to reform their bonds (circles; inset histogram #3: 48.8 ± 4.1 nm, mean±SD, n=117). b) Cartoon representation of the extension events registered on the Cpa trajectory shown in a). Events #1 and #3 show the mechanical extension at 115 pN of thioester-intact Cpa, before cleavage and after reformation, respectively. Event #2 shows the extension after methylamine (MA) cleavage at 24 pN. c) Comparison between the thioester bond reformation (upwards triangles and sigmoidal fit) and the thioester-intact Cpa folding probability (hexagons and sigmoidal fit, from Figure 2c) as a function of the mechanical load. Star symbol indicates the reformation probability obtained at 0 pN from our previous work with AFM11. Data points for reformation are the mean and the error bars are the SD calculated using a jackknife analysis. Reformation registered as the amount of thioester-intact domains after methylamine washout and after a 100 s time-window at the folding/reformation force range (n=13 at 3 pN; n=16 at 4 pN; n=15 at 4.5 pN; n=12 at 5 pN; n=6 at 5.5 pN; n=7 at 6 pN; n=6 at 7 pN).
