Figure 6. Bacterium mobility strategy model based on the allosteric modulation of the Cpa thioester bond by protein folding.
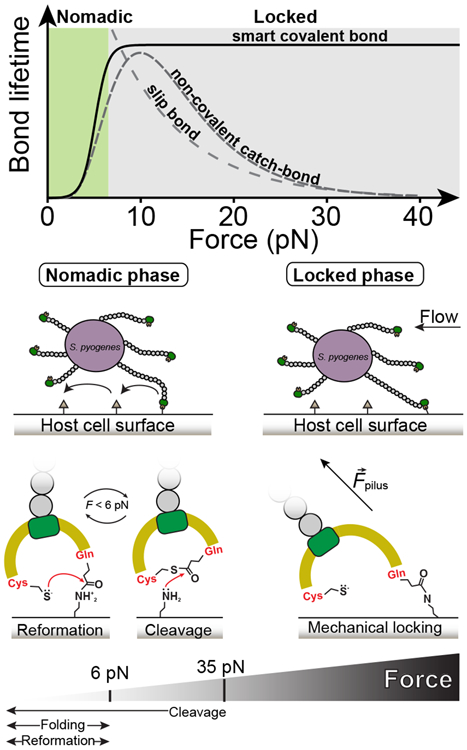
Top graph compares bond lifetimes as a function of the mechanical load for slip bonds, non-covalent catch bonds, and smart covalent bonds (slip bond and catch bond data adapted from64, plotted in arbitrary units). The smart covalent bond lifetime (plotted as the inverse of the thioester bond reformation probability from Figure 4c) is defined as the lifetime of the bond made between the surface ligand and the Gln575 side chain after the nucleophilic cleavage of the thioester bond. While higher loads decrease exponentially the lifetime of slip bonds, in non-covalent catch bonds it increases; however, loads above certain threshold decrease the lifetime. The adhesin-ligand smart covalent bond is allosterically modulated by force, establishing short-lived bonds with surface ligands at low mechanical stress—where thioester bond reformation and cleavage coexist—when the protein is folded, but turning into a long-lived bond that permits the bacterium to remain attached under large mechanical challenges, where thioester bond reformation is prevented. We hypothesize that these smart covalent bonds could allow bacteria to switch between a nomadic mobility phase at low force to a mechanically locked phase at larger loads.
