Extended Data Fig. 1. Cleavage-reformation-cleavage sequence.
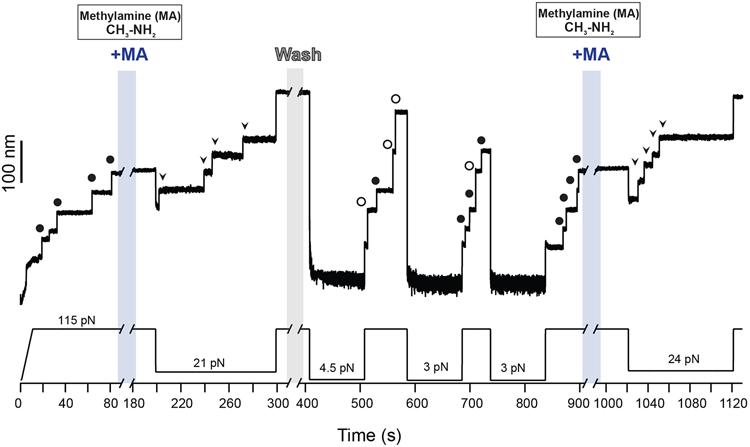
Magnetic tweezers force-clamp trajectory of the Cpa polyprotein. After the unfolding of four thioester-intact Cpa domains at 115 pN (circles, ~49 nm), the buffer is exchanged and the polyprotein is exposed to a solution containing 100 mM methylamine (+MA). At 21 pN, four steps appear which account for the release of the polypeptide sequence trapped by the thioester bonds (arrows). Then, the force is increased again to 115 pN, revealing the complete extension of the molecule. Immediately after, MA is washed out from the fluid chamber and the polyprotein is allowed to fold and reform the thioester bonds for 100 s at 4.5 pN. A 115 pN pulse reveals three ~95 nm steps (empty circles) which correspond with the full extension of Cpa, and one Cpa domain with its thioester bond reformed (circles, ~49 nm). Two more quenches at 3 pN are applied to completely recover the thioester-reformed state in all the four domains, as it can be seen in the 115 pN pulse applied approximately after 800 s of experiment (circles). Then, MA is added again and the force quenched to 24 pN to trigger again the cleavage of the thioester bonds of the polyprotein (arrows).
