Extended Data Fig. 2. Cystamine permanent blocking of Cpa thioester bond reformation.
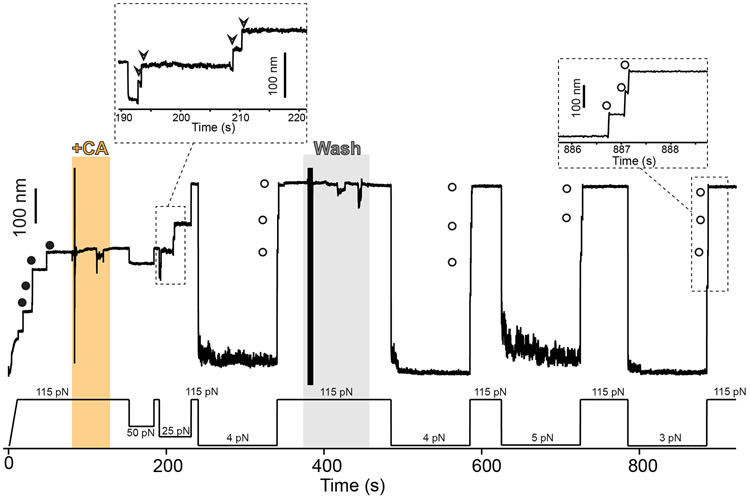
Magnetic tweezers force-clamp trajectory of the Cpa polyprotein. After the unfolding of the thioester-intact Cpa domains at 115 pN (circles), the buffer is exchanged and the polyprotein is exposed to a solution containing 100 mM cystamine (+CA). At 115 pN and at 50 pN, no additional extensions are registered as a consequence of thioester bond cleavage, but a drop in force to 25 pN leads to the appearance of four steps which account for the release of the polypeptide sequence trapped by the thioester bonds (empty arrows in the inset). Then, the force is increased again to 115 pN, revealing the complete extension of the molecule. After 100 s at 4 pN and in the presence of CA, a 115 pN pulse reveals three ~95 nm steps (empty circles) which correspond with the full extension of Cpa. CA is then removed from the solution, and several consecutive 100 s force quenches (at 4, 5, and 3 pN) followed by 115 pN pulses are applied. These cycles reveal that, after CA treatment, Cpa is able to fold but not to reform its thioester bond, as it can be observed from the ~95 nm steps observed (empty circles). After the first 300 s of the experiment, one of the Cpa domains stops folding back as a consequence of oxidative damage65. The disturbances observed in the extension during +CA addition (orange block) and washing (gray block) are originated from the movement of buffer volumes in the liquid cell used in the experiments, which transiently alter the measurement
