Extended Data Fig. 3. TCEP rescues Cpa thioester bond reformation.
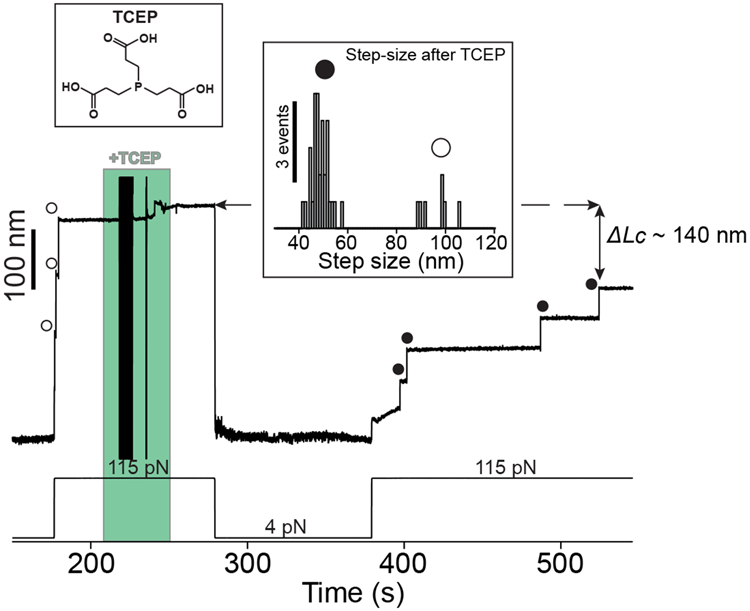
A Cpa polyprotein previously treated with cystamine shows three ~95 nm steps at 115 pN corresponding with the full extension of each of the domains (empty circles). The addition of 10 mM TCEP and 100 s at 4 pN is enough to trigger thioester bond reformation, as it can be observed in the ~49 nm thioester-intact Cpa steps (circles) registered at 115 pN. The fourth domain not observed at the beginning was probably unfolded and its thioester bond intact, since the difference in the final extension between the first 115 pN pulse and the last is ~140 nm, which matches with the expected final extension decrease from three reformation events. Inset histogram shows the two populations of steps observed after TCEP treatment, thioester-intact Cpa (circles, 48.3 ± 3.5 nm, mean±SD, n=32) and thioester-cleaved Cpa (empty circles, 95.7 ± 6.4 nm mean±SD, n=7). The latter full length steps of Cpa after TCEP treatment could be due to cleavage events induced by remaining cystamine which was not completely washed from the experimental liquid cell. The disturbances observed in the extension during +TCEP addition (green block) are originated from the movement of buffer volumes in the liquid cell used in the experiments, which transiently alter the measurement.
