Figure 2: Cross talk between cell death pathways.
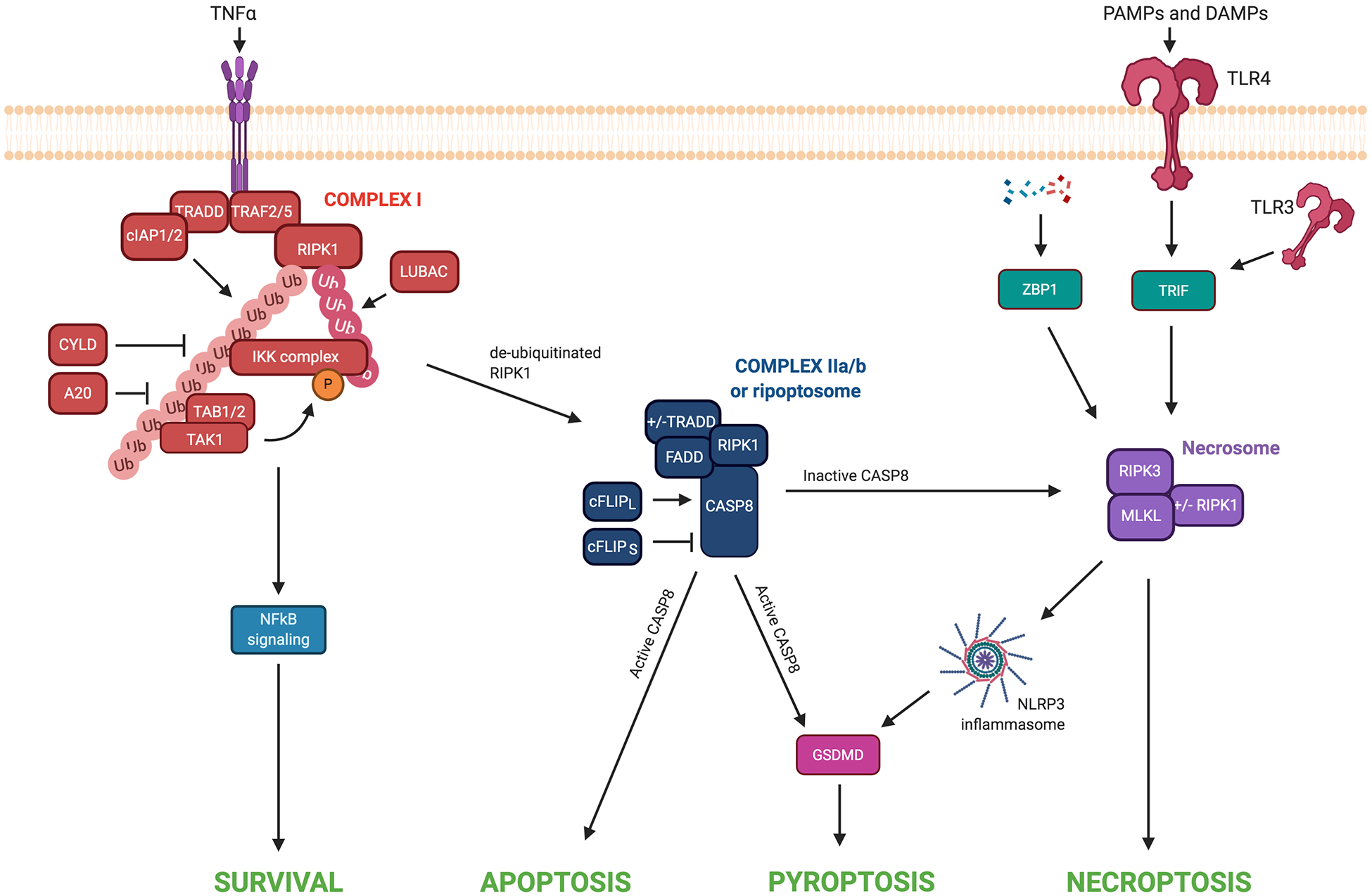
TNFα receptor engagement triggers the formation of Complex I. At this complex, cIAP1/2 and LUBAC ubiquitinate RIPK1, allowing for the recruitment and binding of TAK1, TAB1/2, and IKK complex. Phosphorylation of proteins within the IKK complex by TAK1-TAB2/3 promotes the activation of NF-κB signaling and cell survival. CYLD and A20 can deubiquitinate RIPK1 within Complex I. When RIPK1 is insufficiently ubiquitinated and has not been inactivated by inhibitory phosphorylation events, it translocates to the cytosol where it assembles with additional factors to form Complex II. Within this Complex II, the long isoform of cFLIP activates caspase-8 to prevent necroptotic cell death. Activated caspase-8 can promote activation of the apoptotic signaling cascade and/or cleavage of GSDMD and pyroptotic cell death. Alternatively, the short isoform of cFLIP inhibits caspase-8 activity, allowing for necrosome formation and necroptosis. Alternatively, RIPK3/MLKL can activate the NLRP3 inflammasome to induce pyroptosis. The detection of PAMPs and DAMPs by TLR3/4 leads to the activation of TRIF. When caspase-8 is inhibited, TRIF can promote RIPK3-dependent necroptosis. Intracellular sensing of nucleic acids by ZBP1 promotes necroptotic cell death in specific contexts when RIPK1, FADD, or caspase-8 are deleted or inhibited.
