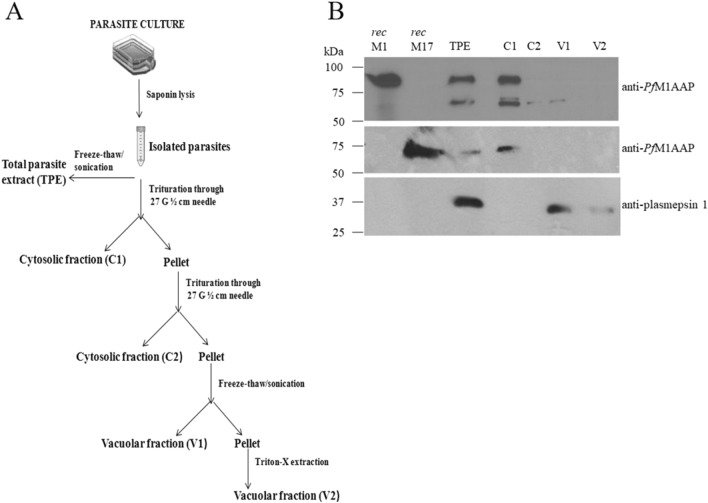
Figure 1.
Distribution of PfM1AAP and PfM17LAP in the P. falciparum cellular compartments. (A) Flowchart depicting the isolation of P. falciparum parasites from host erythrocytes followed by fractionation of cell compartments. Parasites were isolated by saponin lysis of erythrocytes. Total parasite extracts (TPE) were prepared by freeze–thaw and sonication of the parasites in 10 mM Tris–HCl buffer, pH 7.2. Other samples were triturated four times through a syringe needle and centrifuged to obtain the first cytosolic fraction, C1, and a pellet. The pellet was resuspended in 10 mM Tris–HCl buffer, pH 7.2 and triturated/centrifuged to obtain the second cytosolic fraction, C2, and a pellet. This pellet was re-suspended in 10 mM Tris–HCl buffer, pH 7.2, and subjected to four rounds of freeze–thaw treatment followed by centrifugation to obtain a soluble vacuolar fraction, V1, and a pellet. The final detergent-soluble vacuolar fraction, V2, was obtained by incubating the pellet in 0.5% Triton X for 30 min on ice. (B) Representative immunoblots of three biological replicates showing the P. falciparum recombinant (rec) PfM1AAP, rec PfM17LAP, total parasite extract (TPE) and cellular fractions (C1, C2, V1 and V2) probed with anti-PfM1AAP (top panel), anti-PfM17LAP (middle panel), and anti-plasmepsin 1 antibodies (lower panel). Chemiluminescent molecular weight standards are shown on the left.