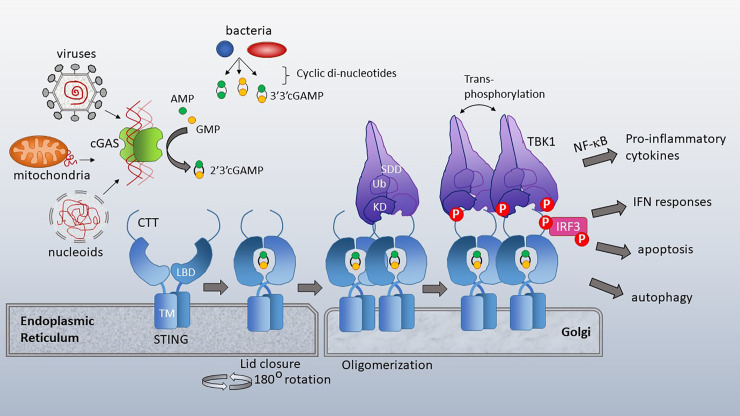
Figure 2.
cGAS and STING activation. Cytosolic dsDNA from viruses, mitochondria or nucleoids formed during nuclear breakdown bind cGAS, triggering its catalytic formation of 2’3’ cGAMP. 2’3’ cGAMP serves as a ligand for STING, which resides in the ER with its ligand binding domain (LBD) facing the cytosol. TM=transmembrane domain. Bacterial cyclic-di-nucleotides, such as cyclic-di-AMP, cyclic-di-GMP and 3’3’ cGAMP also bind STING. Upon ligand binding, the cytosolic domains of STING close over the di-nucleotide ligand and rotate 180 degrees, enabling lateral stacking. STING translocates to the Golgi where it oligomerizes. This oligomerization enhances trans-phosphorylation of the STING CTT (C terminal tail)-associated TBK1 family kinases. TBK1 has a scaffold and dimerization domain (SDD), ubiquitin like domain (Ub) and Kinase domain (KD). Activated TBK1 phosphorylates the STING CTT, enabling recruitment and subsequent phosphorylation of IRF3. TBK1 family kinases also activate signaling pathways leading to NF-κB nuclear translocation. STING activation has diverse immune stimulatory outputs including pro-inflammatory cytokine responses (via NF-κB), interferon responses (via IRF3), apoptosis and autophagy. STING/TBK1 structural cartoon adapted from (51).