Abstract
One aspect of goal-directed behavior, which is known to be impaired in patients with schizophrenia (SZ), is balancing between exploiting a familiar choice with known reward value and exploring a lesser known, but potentially more rewarding option. Despite its relevance to several symptom domains of SZ, this has received little attention in SZ research. In addition, while there is increasing evidence that SZ is associated with chronic low-grade inflammation, few studies have investigated how this relates to specific behaviors, such as balancing exploration and exploitation. We therefore assessed behaviors underlying the exploration–exploitation trade-off using a three-armed bandit task in 45 patients with SZ and 19 healthy controls (HC). This task allowed us to dissociate goal-unrelated (random) from goal-related (directed) exploration and correlate them with psychopathological symptoms. Moreover, we assessed a broad range of inflammatory proteins in the blood and related them to bandit task behavior. We found that, compared to HC, patients with SZ showed reduced task performance. This impairment was due to a shift from exploitation to random exploration, which was associated with symptoms of disorganization. Relative to HC, patients with SZ showed a pro-inflammatory blood profile. Furthermore, high-sensitivity C-reactive protein (hsCRP) positively correlated with random exploration, but not with directed exploration or exploitation. In conclusion, we show that low-grade inflammation in patients with SZ is associated with random exploration, which can be considered a behavioral marker for disorganization. hsCRP may constitute a marker for severity of, and a potential treatment target for maladaptive exploratory behaviors.
Subject terms: Biomarkers, Human behaviour
Introduction
Schizophrenia (SZ) is a complex neuropsychiatric disorder characterized by a wide range of symptoms1. Negative symptoms and disorganization are strongly associated with reduced social and occupational functioning, and are challenging to treat2–5. Therefore, understanding the shared and dissociable mechanisms underlying these symptoms is crucial in increasing our knowledge about the etio-pathophysiology of the disorder and the much needed development of novel treatments.
Negative symptoms and disorganization have been linked to impairments of goal-directed behaviors6,7. Investigating behaviors relevant to these symptom dimensions in SZ with objective, task-based measures that allow the assessment of complex, well-defined behaviors with ecological validity is crucial8,9. The exploration–exploitation trade-off concerns the decision between exploiting a familiar option with known reward value and exploring a lesser known option with more uncertain, but potentially higher reward value. This trade-off may constitute a paradigmatic case for assessing goal-directed behaviors, which have been shown to be impaired in patients with SZ10,11. To study the exploration–exploitation trade-off in a controlled experimental setting, a human computerized task, where the participant has to repeatedly choose between different “slot machines” (bandits) that gradually change their reward values has been developed12,13. Accordingly, participants have to decide when to switch from exploitative to exploratory strategies when aiming to obtain the highest total reward pay-off possible13,14. An optimal balance between exploration and exploitation results in a maximization of the obtained reward, and fulfils the fundamental need of foraging organisms to adapt to a complex and changing world (for review ref. 15).
Few studies have applied exploration–exploitation tasks in patients with SZ and no firm conclusions can be drawn so far: Strauss and colleagues found that patients with SZ show decreased uncertainty-driven directed exploration, which was associated with clinically assessed negative symptoms16. Another study reported that patients with SZ displayed increased novelty-seeking behaviors17. In addition, while several brain regions (midbrain and prefrontal areas) and neurotransmitters (dopamine, noradrenaline, and acetylcholine) have been implicated in mediating exploration–exploitation trade-offs10,15,18,19, the underlying etiological mechanisms remain to be elucidated.
Inflammation has been suggested to be a key mechanism for disturbances of motivation and goal-directed behavior20,21. Preclinical animal models and human studies have shown a close interaction between the peripheral immune system, and the central nervous system in health and the development of neuropsychiatric disorders22,23. Indeed, several studies have reported that patients with SZ display alterations in immune function indexed by cytokines, chemokines, and acute-phase proteins in both blood and cerebral spinal fluid, which are indicative of chronic low-grade inflammation (see ref. 24 for meta-analysis).
Recent studies have started to investigate whether these immune changes correlate with specific behaviors25. With regard to exploration and exploitation, the concept of sickness behavior as an adaptive behavioral response to acute infections is of high relevance26,27. Sickness behavior can be interpreted as a short-term disturbance of exploratory behaviors that could have beneficial energy-saving effects by shifting energy resources toward the immune system to facilitate clearing infections and wound healing. However, in our modern world with fewer infectious challenges and better sanitation, chronic low-grade inflammation might be maladaptive leading to inefficient performance28. To our knowledge, no study has so far investigated the relationship of a pro-inflammatory state with an objective task assessment of exploration–exploitation behavior, despite the high relevance for several symptom domains of SZ. Overall, we hypothesized that patients with SZ will display reduced task outcome based on impairments in balancing between exploration and exploitation behaviors, and that the underlying behavioral impairments are associated with a low-grade, pro-inflammatory state.
Therefore, we first assessed negative, disorganized, and cognitive symptoms in clinically and pharmacologically stable patients with SZ and healthy controls (HC). We then examined exploration vs. exploitation behaviors using a three-armed computerized bandit task, and investigated correlations between overall task performance, directed, and random exploration and exploitation with psychopathological measures. Next, we assessed group differences (SZ vs. HC) in a broad array of cytokines and chemokines in the blood. To investigate and visualize the complex interaction between the individual inflammatory parameters and their relationship with group affiliation (SZ or HC), we used network analysis. Lastly, we investigated the relationships between the immune markers that were different between groups and task-based behaviors.
Results
Sociodemographic data
Sociodemographic and clinical data of the sample are summarized in Table 1. Our SZ sample showed a wide range of negative and disorganized symptoms, and few positive symptoms. Patients with SZ completed fewer years of formal education (p = 0.003) and had a higher body mass index (BMI; p = 0.009) than HC. Nine participants were prescribed clozapine and only one patient used typical antipsychotics. Three patients used lorazepam (up to 1 mg/d) and five patients took antidepressants. The SZ group displayed a significantly lower score in Personal and Social Performance Scale (PSP) as a measure of global level of functioning (p < 0.001). Patients with SZ also displayed a lower cognitive score (p < 0.001).
Table 1.
Sample characteristics.
| HC (n = 19) | SZ (n = 45) | Test statistics | ||
|---|---|---|---|---|
| (t/χ2/U) | p Value | |||
| Age (years) | 32.53 (9.45) | 34.00 (10.47) | t = −0.53 | 0.60 |
| Sex (male/female) | 9/10 | 31/14 | χ2 = 2.64 | 0.10 |
| Formal education (years)a | 14.39 (2.23) | 12.26 (3.88) | U = 227 | 0.003 |
| Number of hospitalizations | — | 5.42 (5.44) | — | — |
| Outpatients/inpatients | — | 24/21 | — | — |
| Smoking (yes/no) | 8/11 | 30/15 | χ2 = 3.34 | 0.068 |
| Number of psychotic episodes | — | 5.31 (5.14) | — | — |
| Illness onset (age, years) | — | 23.91 (6.88) | — | — |
| Illness duration (months) | — | 121.07 (101.28) | — | — |
| Chlorpromazine equivalents (mg/day) | — | 514.67 (444.82) | — | — |
| Clozapine (yes/no) | — | 9/36 | — | — |
| Class of antipsychotic (typical/atypical) | — | 1/43 | — | — |
| Antidepressant medication (yes/no) | — | 5/40 | — | — |
| Lorazepam (yes/no) | — | 3/42 | — | — |
| Body mass index | 23.40 (4.32) | 26.40 (4.66) | U = 250 | 0.009 |
| Psychopathology | ||||
| PANSS negative factorb | — | 15.49 (6.82) | — | — |
| PANSS positive factorc | — | 5.96 (2.70) | — | — |
| PANSS disorganized factord | — | 5.87 (2.48) | — | — |
| PANSS depressive factore | — | 5.33 (2.45) | — | — |
| Cognition | ||||
| Letter–Number Span Test | 20.16 (2.06) | 18.84 (1.93) | t = 2.44 | 0.018 |
| Symbol Coding Test | 81.94 (18.75) | 59.43 (13.28) | t = 4.62 | <0.001 |
| Cognitive scoref | 0 (0.68) | −0.88 (0.70) | t = 4.53 | <0.001 |
| Functioning | ||||
| PSP (total) | 97.05 (4.70) | 51.78 (14.42) | U = 2 | <0.001 |
Data are presented as means (standard deviations). P values < 0.05 are indicated in bold.
BMI body mass index, HC healthy controls, PANSS Positive and Negative Syndrome Scale, PSP Personal and Social Performance Scale, SZ schizophrenia.
aCompulsory education in Switzerland is 9 years.
bNegative factor: N1, N2, N3, N4, N6, G7.
cPositive factor: P1, P3, P5, G9.
dDisorganized factor; P2, N5, G11.
eDepressive factor: P4, P7, G8, G14.
fCognition data have been z-transformed based on the data of the healthy control group for each test separately. The cognitive score was computed as the mean of the z-transformed test scores on subject level.
Patients with SZ display impaired performance in the exploration–exploitation bandit task
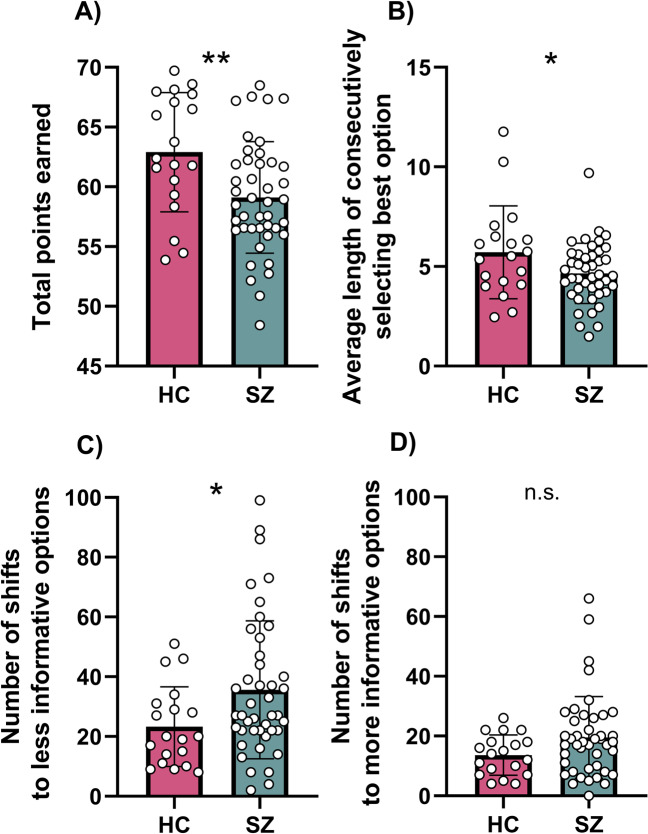
First, the total reward earned from the bandit task was calculated for each group. Patients with SZ obtained significantly fewer points than HC (HC: 62.9 ± 5.0; SZ: 59.1 ± 4.7), t = 2.89 p = 0.005, d = 0.78; Fig. 1A). Both HC and patients with SZ earned significantly more than the simulated random chooser (random choosers: 51.9, HC: p < 0.0001, SZ: p < 0.0001). Thus, even though patients with SZ earned less than HC, individuals with SZ clearly performed better than chance.
Fig. 1. Bandit task performance in patients with schizophrenia compared to healthy controls.
A Patients with SZ earned fewer points in a three-armed bandit task than HC. Compared to HC, patients with SZ patients with SZ displayed B reduced exploitation of the best option, and showed C more random exploration, but D little differences in directed exploration. *p < 0.05, **p < 0.01. n.s. non significant.
In a next step, we aimed to investigate potential reasons for why patients with SZ earned less reward than HC. To do so, we analyzed exploration and exploitation behaviors. We found that exploitation, defined as the continuous selection of the best bandit option, was reduced in patients with SZ (HC: 5.71 ± 2.3; SZ: 4.65 ± 1.5), t = 2.14, p = 0.036, d = 0.55; Fig. 1B). In contrast, there were no significant differences between groups in the length of consecutively selecting other options (p’s > 0.7). We then tested whether there were group differences in exploratory behaviors. SZ patients showed more random exploration (HC = 23.32 ± 13.3, SZ = 35.63 ± 23.1; U = 275.5, p = 0.042, d = 0.65; Fig. 1C), but similar levels of directed exploration (HC = 13.6 ± 6.7, SZ = 19.6 ± 13.7; U = 302, p = 0.104; Fig. 1D). Furthermore, there was a significant negative correlation between random exploration and points earned in the task (r(p) = −0.34, p = 0.025) in patients with SZ.
Next, we performed correlational analyses between the task parameters and psychopathological domains relevant to SZ: negative and depressive symptoms, symptoms of disorganization, and cognitive symptoms. Interestingly, symptoms of disorganization, assessed by the Positive and Negative Syndrome Scale (PANSS) disorganized factor, correlated positively with random exploration (r(s) = 0.31, p = 0.045) and negatively with exploitation (r(s) = −0.36, p = 0.018), the two task parameters that contributed to the decreased overall task performance in SZ patients compared to HC, but not with directed exploration (r(s) = 0.27, p = 0.078). While we observed a significant negative correlation between the PANSS disorganized factor and composite cognitive score (r(s) = −0.33, p = 0.029), task parameters did not correlate with cognitive, positive, negative, or depressive symptoms (Supplementary Table 1). Since antipsychotic medications are known to have an influence on various behaviors and cognition, we correlated chlorpromazine equivalents with task parameters, but did not observe a significant correlation (Supplementary Table 1).
Together, our data show that patients with SZ display impaired task performance as they earned fewer points in the bandit task. Analyzing the underlying behaviors revealed that this deficit is due to increased random exploration and decreased exploitation, which both correlated with the PANSS disorganized factor.
Patients with SZ show a pro-inflammatory phenotype
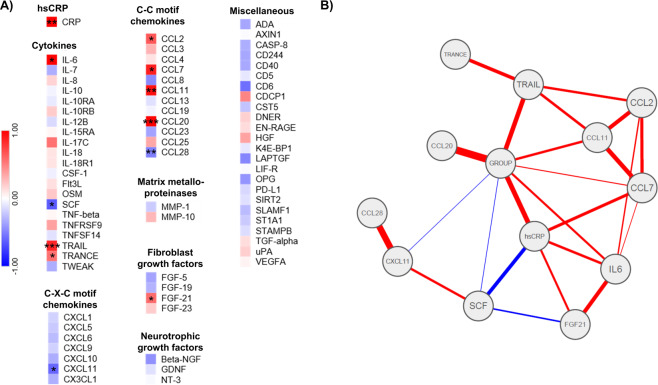
To investigate group differences in blood immune profiles between SZ and HC, we used two different exploratory strategies. First, we investigated group differences of individual immune parameters between HC and patients with SZ. Several immune markers differed between groups, indicative of a pro-inflammatory phenotype in patients with SZ compared to HC (Fig. 2A and Supplementary Table 3): high-sensitivity C-reactive protein (hsCRP; U = 243.5, p = 0.007, d = 0.96), interleukin (IL)-6 (t = −2.31, p = 0.025, d = 0.74), tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL; t = −4.16, p < 0.001, d = 1.05), TNF-related activation-induced cytokine TRANCE (TRANCE; t = −2.09, p = 0.041, d = 0.59), fibroblast growth factor 21 (FGF-21; t = −2.19, p = 0.033, d = 0.61), chemokine ligand (CCL) 2 (t = −2.05, p = 0.045, d = 0.61), CCL7 (t = −2.23, p = 0.030, d = 0.71), CCL11 (t = −2.74, p = 0.008, d = 0.83), and CCL20 (U = 158.5, p < 0.001, d = 1.02) were increased in the SZ group, while stem cell factor (t = 2.13, p = 0.038, d = 0.62), CXCL11 (U = 223, p = 0.026, d = 0.56), and CCL28 (U = 183.5, p = 0.003, d = 0.48) were decreased.
Fig. 2. Peripheral blood inflammatory markers.
A Heat maps of standardized z-scores of inflammatory markers. Red indicates increased expression and blue decreased expression (patients with schizophrenia vs. healthy control subjects). B Network analysis. The thickness of an edge corresponds to its weight. Lines indicate positive (red) or negative (blue) partial correlation between two nodes. *p < 0.05, **p < 0.01, ***p < 0.001. For a list of abbreviations see Supplementary Table 2.
hsCRP (p = 0.022), TRAIL (p = 0.001), CCL20 (p = 0.003), CCL7 (p = 0.027), and CCL11 (p = 0.010) remained significantly different after controlling for age, sex, and BMI.
We then performed network analysis of the inflammatory markers that were significantly different between the two groups. Given that cytokines and other pro-inflammatory markers are known to influence each other reciprocally, network analysis can reveal distinct associations between group affiliation and the included proteins. Controlling for the associations with all other variables in the network, we found group affiliation to be uniquely correlated with several inflammatory markers, most strongly with CCL20, TRAIL, and hsCRP (Fig. 2B and Supplementary Figs. 3–7).
Taken together, several pro-inflammatory cytokines and chemokines were elevated in patients with SZ compared to HC, indicative of a pro-inflammatory state. Across all the analyses, hsCRP, CCL20, and TRAIL were most strongly associated with group.
Random but not directed exploration is associated with increased hsCRP
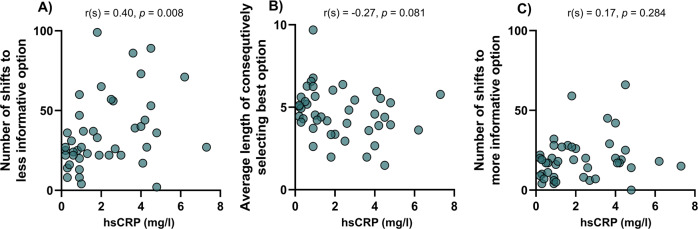
Finally, we correlated bandit task behaviors with TRAIL, CCL20, and hsCRP, the immune parameters that were most strongly associated with group across the analyses, in patients with SZ. To control for multiple comparisons, significance level was set at p < 0.017 (0.05/3 parameters).
We found a significant positive correlation of hsCRP with random exploration (r(s) = 0.40, p = 0.008; Fig. 3A), but not exploitation (r(s) = −0.27, p = 0.081; Fig. 3B), or directed exploration (r(s) = 0.17, p = 0.284; Fig. 3C). The correlation between random exploration and hsCRP remained significant after controlling for age, BMI, sex, smoking, cognition, chlorpromazine equivalents, PSP, years of formal education, number of hospitalizations, number of psychotic episodes, illness duration, and age at illness onset (r = 0.40, p = 0.026). There were no correlations of CCL20 or TRAIL with task parameters (Supplementary Table 4).
Fig. 3. Associations between task performance and high-sensitivity C-reactive protein in patients with schizophrenia.
Within patients with SZ, correlation of hsCRP with A random exploration was significant, in contrast to correlation with B exploitation or C directed exploration.
Discussion
In the present study, we investigated the link between shifted balancing of exploration vs. exploitation using an objective task-based measure, and inflammatory parameters in patients with SZ. Compared to HC, patients with SZ showed reduced performance in the bandit task. Furthermore, we demonstrated that reduced task performance was associated with increased random exploration in SZ. Random exploration correlated with the acute-phase protein hsCRP.
To our knowledge, the specific increase in random exploration associated with reduced overall performance has not been previously reported for patients with SZ. However, in the study of Martinelli and colleagues, patients with SZ showed increased novelty seeking on a different version of the three-armed bandit task17. Although the task and the analysis are not directly comparable, their findings are consistent with ours as novelty seeking and random exploration tap into similar functions. In addition, several studies using simpler tasks have shown that patients with SZ display impaired ability to keep their best decisions and instead more often show “win-shift” behaviors, which is in line with our finding of reduced exploitation29,30. In contrast to findings from our study, Strauss and colleagues showed, using a “temporal utility integration task” that, compared to HC, patients with SZ have decreased uncertainty-driven directed exploration, an effect that was most pronounced in patients with SZ with high levels of negative symptoms16. Further, directed exploration was negatively associated with anhedonia in SZ16. Although the use of different tasks makes direct comparisons difficult, it is interesting that in the present study patients with SZ did not show differences in directed exploration or correlations of negative symptoms with task parameters.
Instead, we observed a positive correlation between symptoms of disorganization and random exploration. Compared to other symptom domains, symptoms of disorganization have been the least studied, but are closely linked to functional outcomes2,31. Specifically, they have been associated with lack of insight32, lower quality of life33, and impaired long-term functioning34. In addition, several studies have shown that symptoms of disorganization are related to symptoms of cognition31,35. In line with these findings, we show a negative correlation between PANSS disorganized factor and cognitive score. However, the increased random exploration cannot be solely explained by cognition because we did not find a correlation between cognitive score and task behaviors. Taken together, our data suggest that exploratory behaviors assessed by the bandit task are less relevant to negative symptoms, but are more related to symptoms of disorganization, i.e., impairment of integrating complex inputs about a changing environment into an organized, efficient process.
The neural mechanisms underlying exploration–exploitation trade-offs and random exploration in particular have not been fully elucidated yet. Several neurotransmitters, including dopamine, acetylcholine, and norepinephrine have been suggested to modulate exploration–exploitation trade-offs10. While the neuromodulator dopamine has been consistently implicated in balancing exploration with exploitation36–38, the underlying etiological causes of this dopamine dysregulation are not well understood. There is a growing body of evidence indicating SZ is associated with chronic low-grade inflammation25,39,40 and that pro-inflammatory cytokines can directly influence dopamine neurotransmission20. We therefore investigated if a pro-inflammatory state is associated with the observed task performance deficits and in extension the hypothesized hypodopaminergic state. In line with previous research25, patients with SZ showed a pro-inflammatory state. Across all the analyses investigating group differences of inflammatory parameters, CCL20, TRAIL, and hsCRP were most strongly associated with SZ group affiliation. In addition, hsCRP was positively correlated with random exploration, the task behavior that contributed to decreased overall task performance. Taken together, the current data suggest that patients with SZ show increased pro-inflammatory markers compared to HC, and that hsCRP is associated with increased inefficient, random exploration.
At present, we cannot be certain about the exact mechanisms of how CRP causally affects exploratory behavior, because of the numerous functions of this acute-phase protein in the body’s immune response41. Its production predominantly takes place in hepatocytes where it is induced by pro-inflammatory cytokines, mainly IL-6 and TNF-α (ref. 42). While CRP is widely used in clinical practice to detect and monitor the course of a variety of inflammatory and immune processes, much less is known about its function43. One of the major roles of CRP is the activation of the classical complement pathway44. In circulation, the complement system interacts with pathogens to mark them for destruction by phagocytes45. The literature regarding findings in patients with SZ is inconclusive: while some studies have shown increased peripheral complement protein concentration in patients with SZ46,47, a recent meta-analysis has concluded that the evidence regarding complement system dysregulation is mixed48. In addition, recent evidence has suggested that the complement cascade is involved in several central nervous system processes, including neurogenesis and synaptic pruning, and has specifically been implicated in SZ49,50. Further studies are needed to investigate how peripheral complement levels are linked to the central nervous system and pathologies relevant to SZ, especially given that complements do not cross an intact blood–brain barrier (BBB)51. An additional potential role of CRP with relevance to SZ could be its ability to facilitate recruitment of peripheral leukocytes to the brain. Recent studies have indicated some evidence that a subgroup of patients with SZ display signs of leukocyte infiltration into the prefrontal cortex52. In vitro studies have shown that CRP promotes CCL2 mediated chemotaxis through the upregulation of chemokine receptor (CCR)2 on monocytes53. Although preliminary, this could constitute an interesting mechanism. While CRP itself does not cross the BBB, peripheral CRP was shown to directly increase the permeability of the BBB by reducing tight junction proteins54. This increased BBB permeability could facilitate the access of potentially neurotoxic substances, such as pro-inflammatory cytokines, peripheral leukocytes, or autoantibodies to the brain25,55.
In contrast to CRP, we did not find an association between TRAIL or CCL20, and exploratory behavior although they were increased in patients with SZ compared to HC. TRAIL (or tumor necrosis factor (ligand) superfamily, member 10 (TNFSF10)) is a member of the TNF cytokine superfamily56. One of the major functions of TRAIL is the induction of apoptosis57,58. Since apoptotic processes of neurons have been implicated in the pathophysiology of SZ59, TRAIL induced neuronal apoptosis could constitute an interesting potential disease mechanism. However, further studies investigating a causal mechanism are warranted. Chemokine (C–C motif) ligand 20 (CCL20), also known as macrophage inflammatory protein 3α, is expressed in various human tissues and immune cells60 and is the only chemokine ligand to the chemokine receptor CCR661. Multiple immune cells express CCR6, including both pro-inflammatory Th17 and regulatory T cells62. Dysregulation of peripheral T cells has been associated with SZ and CCL20 could potentially play a role in this dysregulation, but direct evidence for this link still needs to be elucidated63–67.
There are several limitations that should be considered when interpreting the results of the current study: first, patients were medicated, and although a recent meta-analysis has not found an effect of antipsychotics on CRP25, smaller studies have shown associations between antipsychotic medication and CRP68, so a confounding effect of certain classes of antipsychotics on both behavior and metabolism cannot be excluded. Moreover, to causally test the hypothesis that peripheral inflammatory mediators can indeed influence neurotransmitter levels leading to behavioral changes, future studies directly measuring dopamine and pharmacologically blocking dopamine receptors in combination with experimentally induced immune challenges in HC, patients with SZ, and preclinical models are needed.
Taken together, the present study suggests that patients with SZ are more likely to randomly explore than HC and that this behavior is associated with symptoms of disorganization. Importantly, this inefficient form of exploratory behavior is associated with a pro-inflammatory state. Further studies are needed to elucidate the underlying mechanism of how CRP leads to the neurobiological changes resulting in the observed behavioral alterations. Nevertheless, our data suggest that hsCRP could be a marker for the severity and treatment course of and potentially a therapeutic target for maladaptive exploratory behaviors in SZ, and potentially other neuropsychiatric disorders.
Methods
Participants
Forty five patients (24 outpatients and 21 inpatients) meeting the DSM-V (ref. 69) criteria for SZ and 19 HC subjects were recruited. Patients were recruited from outpatient and inpatient units of the Psychiatric Hospital of the University of Zurich and affiliated institutions, HC were recruited from the community via advertisement. More patients than controls were recruited to have adequate power for the correlational analyses (see below). Two patients did not complete the bandit task (due to fatigue) and are therefore only included in the group comparison of immune markers. Diagnoses were confirmed by conducting the Mini-International Neuropsychiatric Interview70. All patients were clinically stable and under a stable dose of medication for at least 2 weeks prior to testing. Inpatients were at the end of their hospitalization, and engaged in a multimodal therapy program and activities outside the hospital. The average duration of hospitalization for patients with SZ in Swiss psychiatric hospitals is longer than in most other countries, so the majority of patients would have been treated as outpatients in other health care systems71,72. The inclusion age was between 18–65 years. We excluded patients with any other than the above mentioned DSM-IV Axis I disorder (including major depressive disorders), benzodiazepines (except lorazepam equivalents of 1 mg or less per day), and acute psychotic symptoms. Participants with any alcohol use disorder based on lifetime criteria and participants with current cannabis abuse or dependency, or with any other substance abuse were excluded. Chlorpromazine equivalents were calculated according to ref. 73. HC were excluded if any neuropsychiatric diagnosis was present in the structured Mini-International Neuropsychiatric Interview. In both groups, participants were excluded if they had a history of head injury or any autoimmune or chronic inflammatory disorder, or if they took any pain medication or anti-inflammatory drugs at least 1 week prior to testing (assessed by detailed questionnaire and medical records where available). Furthermore, participants were not included in the study if they had a history of any known acute inflammation 2 weeks prior to testing. All participants gave written informed consent and the project was approved by the Ethics Committee of the Canton of Zurich.
Assessment of psychopathology and cognition
On the day of testing, study participants were first screened for the presence of symptoms of an acute infection and their fasting state was verified by a questionnaire. After the blood draw (see below), their diagnosis was confirmed with the Mini-International Neuropsychiatric Interview70. Then, psychopathological symptoms were assessed with the PANSS74. The PANSS factor scores were calculated according to the five-factor model of Wallwork and colleagues75 The negative symptom factor was calculated based on items N1, N2, N3, N4, N6, G7, the positive factor computed from items P1, P3, P5, G9, the disorganized factor was calculated based on items P2, N5, G11, and the depressive factor computed from items P4, P7, G8, and G14. Cognition was assessed with the Brief Neurocognitive Assessment (BNA)76, which was shown to be highly correlated with the MATRICS Consensus Cognitive Battery77 and has similarly good psychometric properties78. With the BNA, a cognitive score is computed for each participant by combining results from the Letter–Number span (sequencing) (LNS) test and the Symbol Coding Test (SCT)76. In the LNS, participants are read a sequence of letters and digits which they then have to report back, listing first the numbers in ascending order, and then the letters in alphabetical order78. This test was shown to measure working memory capacity and attention79. The SCT allows to measure processing speed; participants are required to assign in a given time as many numbers from an item-number key to the corresponding item78. Results of the subtests were then z-transformed based on the data of the HC group and a composite cognitive score was computed as the mean of the z-transformed test scores. The global level of functioning was assessed using the PSP80.
Bandit task
For assessing exploratory vs. exploitative behavior, the participants performed a computerized virtual slot machine (bandit) task (Supplementary Fig. 1). The procedure is an adapted version of a recently described bandit task14. Briefly, participants were shown three virtual slot machines depicted by three blue boxes on a screen between which they had to choose repeatedly by pressing the arrow keys on the keyboard. After each choice, the points they won, ranging from 1 to 100, were displayed. Participants were instructed to collect as many points as possible to maximize their gain. In foraging situations, like in this bandit task, decision makers need to balance exploitation with exploration. They were informed that the points paid out by the slot machines oscillated randomly and independently over trials, so they would have to choose at any given point between exploiting the currently used bandit and exploring the other two options to maximize gain. Unlike standard slots, the mean payoffs changed randomly and independently from trial to trial, with subjects finding information about the current worth of a slot only through sampling it13. Three brief written questions and a short practice trial to confirm the comprehension of the task took place before the actual task began. The payouts for each bandit were generated with a decaying Gaussian random walk, resulting in points paid off varying noisily around three different means. Reward of the selected option was displayed on the computer screen after each choice. If participants took too much time (5 s) deciding between the slot machines, a window popped up asking them to choose a machine. Participants completed 200 trials. At the end, five trials were randomly selected and the participants received the mean of the corresponding gains. To test whether participants performed better than chance, we simulated random choosers (equal chance to select each bandit) 1000 times for each participant’s reward structure. The average total reward earned by the random choosers was 51.90 points. We tested whether control participants and participants with SZ performed better or worse than the average of random choosers. To avoid any unnecessary assumptions about the actual implementation of psychological functions, we performed model-free analyses. We defined the best, second-best and worst options as the options with the highest, second highest, and lowest expected value in the current trial based on the objective reward structure of the task. Continuously selecting the best option was defined as exploitation. There was a significant correlation between the average length of consecutively selecting the best option and points earned in the task (r(s) = 0.53, p < 0.001), further validating our measure of exploitation. Because the points paid out by the slot machines oscillated randomly and independently over trials, too strong exploitation with result in nonoptimal behavior, where people have insufficient information about the current reward structure. Ideally, decision makers should balance exploitation with exploration. To further disentangle different forms of exploratory behaviors, we distinguished directed from random exploration based on the uncertainty associated with each option36,81,82. The less often a given bandit has been chosen, the less information the decision maker has about the bandit. Accordingly, directed exploration corresponds to choosing the option that provides most information for future decisions, and can be viewed as goal-directed uncertainty reduction. In contrast, random exploration can be interpreted as a noisy behavior, which provides little or no extra information. In our task, directed exploration corresponds to a shift to the least selected option while shifts to more often selected options correspond to random exploration. A less well-established alternative definition of directed and random exploration is based on the value of each option, with value-based directed exploration defined as switching from the best option to the second-best option. In the supplemental material, we show that the main findings of the present study remained qualitatively the same using this alternative definition (Supplementary Fig. 2).
Blood samples and processing
Blood was drawn between 8 and 10 a.m. on the day of testing. Study participants were instructed to fast for at least 8 h prior to the blood draw and this was verified by a questionnaire on the day of testing. To obtain plasma for the Olink panel, blood was drawn into ethylenediaminetetraacetic acid tubes (Sarstedt, Switzerland), centrifuged for 15 min at 1500 × g and plasma was frozen at −80 °C. hsCRP was measured in serum: blood was collected into a silica and gel containing tube (BD Vacutainer).
hsCRP
hsCRP was measured in participant serum samples by immunoturbidimetry on Abbott Architect c16000 or c8000, at Unilabs Medical Analytics in Duebendorf/Zurich, Switzerland.
Cytokines and chemokines
Proteins were measured using the Olink® Inflammation panel (Olink Proteomics AB, Uppsala, Sweden) according to the manufacturer’s instructions. The Proximity Extension Assay technology used for the Olink protocol is described in detail in ref. 83. In brief, pairs of oligonucleotide-labeled antibody probes bind to their targeted protein, and if the two probes are brought in close proximity the oligonucleotides will hybridize in a pair-wise manner. The addition of a DNA polymerase leads to a proximity-dependent DNA polymerization event, generating a unique PCR target sequence. The resulting DNA sequence is subsequently detected and quantified using a microfluidic real-time PCR instrument (Biomark HD, Fluidigm). Data are then quality-controlled and normalized using an internal extension control and an inter-plate control, to adjust for intra- and inter-run variation. The final assay readout is presented in normalized protein expression values, which is an arbitrary unit on a log2-scale, where a high value corresponds to a higher protein expression. Detection limits are available on the manufacturer’s website (www.olink.com). In total, 91 proteins were measured with an intra- and inter-assay coefficient of variance of <10%. We excluded 18 proteins due to >80% values below the limit of detection (LOD). Values below the LOD were replaced with the LOD for the specific assay. Five participants (two HC and three SZ) were excluded because their samples failed quality control.
Data analysis
All variables were tested for normal distribution using the Shapiro-Wilk test. If variables were normally distributed, potential group differences were examined using two-sided Students t tests. To investigate correlations between inflammatory parameters and task variables/psychopathology, Pearson correlation coefficients r(p) were calculated. If data were not normally distributed, we instead used Mann–Whitney U tests and Spearman correlation coefficients r(s). Heat maps of inflammatory markers are based on standardized scores that were calculated as: (value of SZ (individual sample value) − mean of HCs)/standard deviation of HCs). The level of significance was set at p < 0.05. To investigate group differences in inflammatory markers between HC and patients with SZ, we used an exploratory approach using uncorrected test statistics and network analysis (see below). Correlations between immune markers and task behavior was controlled for multiple comparisons using the procedure proposed by Benjamini and Hochberg84. General linear models were used to test for the effects of covariates on group differences and partial correlations were calculated when controlling for covariates in the correlational analyses. Effect size was assessed by calculating Cohen’s d. Statistical analyses were computed with SPSS version 25 (IBM Corp., SPSS Inc., Chicago IL, USA), Stata version 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP), Matlab version R2018a (The MathWorks, Inc., Natick, Massachusetts, United States), R (Version 3.4.0 (ref. 85), and GraphPad Prism software (GraphPad Software Inc.). Heat maps were created using the online tool Morpheus (https://software.broadinstitute.org/morpheus).
Network analysis
Network analyses followed a four-step procedure: (i) data preparation: all variables for which the t test for differences in blood levels of investigated inflammation markers between patients with SZ and HC had a p value <0.05 before correction for multiple testing were included in the network. (ii) Network estimation and visualization: the estimated network is a Gaussian Graphical Model, in which the nodes represent the included variables. Edges between these variables can be interpreted as undirected, weighted partial correlations. Partial correlations can be thought of as the “unique” correlation between two nodes after controlling for the correlation with all other nodes86. We then visualized the network, with the thickness of an edge corresponding to its weight and with the color indicating a positive (red) or negative (blue) partial correlation between two nodes. Placement of the nodes for the visualization was based on the Fruchterman–Reingold algorithm87. (iii) Network characterization: we estimated centrality indices, which are a relative marker for the influence of a node on the network as a whole (for detail see ref. 88). (iv) Network accuracy and stability: using current state of the art procedures, we calculated 95% confidence intervals for the edge weights and difference test for centrality and edge weights. All network analyses were carried out in R (version 3.6.1 (ref. 85)) using the package bootnet86. Networks were visualized using qgraph89. Detailed information about network analysis are provided in the SI.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Supplementary information
Acknowledgements
This work was supported by a Postdoc.Mobility Fellowship of the Swiss National Science Foundation (F.C.), a Walter and Gertrud Siegenthaler Postdoctoral Fellowship (F.C.), and project support by the Hartmann Mueller Foundation (F.C.), Kurt und Senta Herrmann Stiftung (F.C.), and the Olga Mayenfisch Stiftung (M.H.). F.K. was supported by an Early.Postdoc Mobility Fellowship of the Swiss National Science Foundation. P.N.T. acknowledges support by the Swiss NSF (Grants PP00P1 150739 and 100014_165884). T.S. was supported by the Forschungskredit of the University of Zurich (FK-19-048). S.K. was funded by the Swiss National Science Foundation (10001CL_169783).
Author contributions
Study concept and design: F.C. and S.K. Acquisition, analysis, or interpretation of data: F.C., F.K., K.G., H.-K.C., A.R.B., T.S., R.S., M.N.H., and P.N.T. Manuscript writing: F.C., P.T., and S.K. Funding: F.C., M.N.H., E.S., and S.K. All authors edited and approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41537-020-00133-0.
References
- 1.Kahn RS, et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- 2.Rocca P, et al. Disorganization and real-world functioning in schizophrenia: results from the multicenter study of the Italian Network for Research on Psychoses. Schizophr. Res. 2018;201:105–112. doi: 10.1016/j.schres.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 4.Foussias G, et al. Prediction of longitudinal functional outcomes in schizophrenia: the impact of baseline motivational deficits. Schizophrenia Res. 2011;132:24–27. doi: 10.1016/j.schres.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Strauss GP, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J. Psychiatr. Res. 2013;47:783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RG, Pluck G. Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends Neurosci. 2000;23:412–417. doi: 10.1016/S0166-2236(00)01626-X. [DOI] [PubMed] [Google Scholar]
- 7.Sitnikova T, Goff D, Kuperberg GR. Neurocognitive abnormalities during comprehension of real-world goal-directed behaviors in schizophrenia. J. Abnorm. Psychol. 2009;118:256–277. doi: 10.1037/a0015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luther L, Fischer MW, Firmin RL, Salyers MP. Clarifying the overlap between motivation and negative symptom measures in schizophrenia research: a meta-analysis. Schizophr. Res. 2019;206:27–36. doi: 10.1016/j.schres.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui I, et al. Objective assessment of exploratory behaviour in schizophrenia using wireless motion capture. Schizophr. Res. 2018;195:122–129. doi: 10.1016/j.schres.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Addicott MA, Pearson JM, Sweitzer MM, Barack DL, Platt ML. A primer on foraging and the explore/exploit trade-off for psychiatry research. Neuropsychopharmacology. 2017;42:1931–1939. doi: 10.1038/npp.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratt SC, Sumpter DJ. A tunable algorithm for collective decision-making. Proc. Natl Acad. Sci. USA. 2006;103:15906–15910. doi: 10.1073/pnas.0604801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gittins JC. Bandit processes and dynamic allocation indices. J. R. Stat. Soc. Ser. B (Methodol.) 1979;41:148–177. [Google Scholar]
- 13.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja Beharelle A, Polania R, Hare TA, Ruff CC. Transcranial stimulation over frontopolar cortex elucidates the choice attributes and neural mechanisms used to resolve exploration-exploitation trade-offs. J. Neurosci. 2015;35:14544–14556. doi: 10.1523/JNEUROSCI.2322-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JD, McClure SM, Yu AJ. Should I stay or should I go? How the human brain manages the trade-off between exploitation and exploration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007;362:933–942. doi: 10.1098/rstb.2007.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss GP, et al. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol. Psychiatry. 2011;69:424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinelli C, Rigoli F, Averbeck B, Shergill SS. The value of novelty in schizophrenia. Schizophr. Res. 2018;192:287–293. doi: 10.1016/j.schres.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 20.Treadway MT, Cooper JA, Miller AH. Can’t or won’t? Immunometabolic constraints on dopaminergic drive. Trends Cogn. Sci. 2019;23:435–448. doi: 10.1016/j.tics.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 2018;98:477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cathomas F, Murrough JW, Nestler EJ, Han MH, Russo SJ. Neurobiology of resilience: interface between mind and body. Biol. Psychiatry. 2019;86:410–420. doi: 10.1016/j.biopsych.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes BS, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol. Psychiatry. 2016;21:554–564. doi: 10.1038/mp.2015.87. [DOI] [PubMed] [Google Scholar]
- 26.Dantzer R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes M, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culbreth AJ, Gold JM, Cools R, Barch DM. Impaired activation in cognitive control regions predicts reversal learning in schizophrenia. Schizophr. Bull. 2016;42:484–493. doi: 10.1093/schbul/sbv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy LF, Waltz JA, Green MF, Wynn JK, Horan WP. Probabilistic reversal learning in schizophrenia: stability of deficits and potential causal mechanisms. Schizophr. Bull. 2016;42:942–951. doi: 10.1093/schbul/sbv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventura J, Thames AD, Wood RC, Guzik LH, Hellemann GS. Disorganization and reality distortion in schizophrenia: a meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophr. Res. 2010;121:1–14. doi: 10.1016/j.schres.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteiro LC, Silva VA, Louza MR. Insight, cognitive dysfunction and symptomatology in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258:402–405. doi: 10.1007/s00406-008-0809-8. [DOI] [PubMed] [Google Scholar]
- 33.Sigaudo M, et al. Quality of life in stable schizophrenia: the relative contributions of disorganization and cognitive dysfunction. Schizophr. Res. 2014;153:196–203. doi: 10.1016/j.schres.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Evans JD, et al. Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophr. Res. 2004;70:331–342. doi: 10.1016/j.schres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Minor KS, Lysaker PH. Necessary, but not sufficient: links between neurocognition, social cognition, and metacognition in schizophrenia are moderated by disorganized symptoms. Schizophr. Res. 2014;159:198–204. doi: 10.1016/j.schres.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat. Neurosci. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gershman SJ, Uchida N. Believing in dopamine. Nat. Rev. Neurosci. 2019;20:703–714. doi: 10.1038/s41583-019-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphries M, Khamassi M, Gurney K. Dopaminergic control of the exploration-exploitation trade-off via the basal ganglia. Front. Neurosci. 2012;6:9. doi: 10.3389/fnins.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin. Schizophr. Relat. Psychoses. 2014;7:223–230. doi: 10.3371/CSRP.MICU.020813. [DOI] [PubMed] [Google Scholar]
- 40.Fond G, et al. Chronic low-grade peripheral inflammation is associated with ultra resistant schizophrenia. Results from the FACE-SZ cohort. Eur. Arch. Psychiatry Clin. Neurosci. 2019;269:985–992. doi: 10.1007/s00406-018-0908-0. [DOI] [PubMed] [Google Scholar]
- 41.Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J. Exp. Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luan Y-Y, Yao Y-M. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 2018;9:1302–1302. doi: 10.3389/fimmu.2018.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J. Immunol. 1974;112:2135–2147. [PubMed] [Google Scholar]
- 45.Brown JS, et al. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl Acad. Sci. USA. 2002;99:16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos Sória L, Moura Gubert C, Ceresér KM, Gama CS, Kapczinski F. Increased serum levels of C3 and C4 in patients with schizophrenia compared to eutymic patients with bipolar disorder and healthy. Rev. Bras. Psiquiatr. 2012;34:119–120. doi: 10.1590/S1516-44462012000100022. [DOI] [PubMed] [Google Scholar]
- 47.Laskaris L, et al. Investigation of peripheral complement factors across stages of psychosis. Schizophr. Res. 2019;204:30–37. doi: 10.1016/j.schres.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Mongan D, et al. Peripheral complement proteins in schizophrenia: a systematic review and meta-analysis of serological studies. Schizophr Res. 2020;222:58–72. doi: 10.1016/j.schres.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 50.Sekar A, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan BP. Complement in the pathogenesis of Alzheimer’s disease. Semin. Immunopathol. 2018;40:113–124. doi: 10.1007/s00281-017-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai HQ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol. Psychiatry. 2020;25:761–775. doi: 10.1038/s41380-018-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han KH, et al. C-reactive protein promotes monocyte chemoattractant protein-1–mediated chemotaxis through upregulating CC chemokine receptor 2 expression in human monocytes. Circulation. 2004;109:2566–2571. doi: 10.1161/01.CIR.0000131160.94926.6E. [DOI] [PubMed] [Google Scholar]
- 54.Elwood E, Lim Z, Naveed H, Galea I. The effect of systemic inflammation on human brain barrier function. Brain Behav. Immun. 2017;62:35–40. doi: 10.1016/j.bbi.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell BM, Charych E, Lee AW, Moller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front. Neurosci. 2014;8:12. doi: 10.3389/fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Miguel D, Lemke J, Anel A, Walczak H, Martinez-Lostao L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016;23:733–747. doi: 10.1038/cdd.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan G, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 58.MacFarlane M, et al. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 1997;272:25417–25420. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- 59.Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29:846–858. doi: 10.1016/j.pnpbp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 60.Power CA, et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J. Exp. Med. 1997;186:825–835. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baba M, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 1997;272:14893–14898. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- 62.Comerford I, et al. An immune paradox: how can the same chemokine axis regulate both immune tolerance and activation?: CCR6/CCL20: a chemokine axis balancing immunological tolerance and inflammation in autoimmune disease. BioEssays. 2010;32:1067–1076. doi: 10.1002/bies.201000063. [DOI] [PubMed] [Google Scholar]
- 63.Debnath M. Adaptive immunity in schizophrenia: functional implications of T cells in the etiology, course and treatment. J. NeuroImmune Pharmacol. 2015;10:610–619. doi: 10.1007/s11481-015-9626-9. [DOI] [PubMed] [Google Scholar]
- 64.Drexhage RC, et al. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int. J. Neuropsychopharmacol. 2011;14:746–755. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
- 65.Ding M, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;51:78–82. doi: 10.1016/j.pnpbp.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Egea E, et al. Peripheral immune cell populations associated with cognitive deficits and negative symptoms of treatment-resistant schizophrenia. PloS ONE. 2016;11:e0155631. doi: 10.1371/journal.pone.0155631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly DL, et al. Increased circulating regulatory T cells in medicated people with schizophrenia. Psychiatry Res. 2018;269:517–523. doi: 10.1016/j.psychres.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fond G, et al. Relationships between low-grade peripheral inflammation and psychotropic drugs in schizophrenia: results from the national FACE-SZ cohort. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268:541–553. doi: 10.1007/s00406-017-0847-1. [DOI] [PubMed] [Google Scholar]
- 69.Association, A. P. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th edn, xliv, 947 (American Psychiatric Association, 2013).
- 70.Lecrubier, Y., Weiller, E. & Herugeta, T. Mini International Neuropsychiatric Interview German Version 5.0. 0 (Psychiatrischen Universitätsklinik München, München 1999).
- 71.Kirschner M, et al. Shared and dissociable features of apathy and reward system dysfunction in bipolar I disorder and schizophrenia. Psychological Med. 2020;50:936–947. doi: 10.1017/S0033291719000801. [DOI] [PubMed] [Google Scholar]
- 72.Hartmann MN, et al. Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr. Bull. 2015;41:503–512. doi: 10.1093/schbul/sbu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/JCP.v64n0607. [DOI] [PubMed] [Google Scholar]
- 74.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br. J. Psychiatry Suppl. 1989;155:59–67. doi: 10.1192/S0007125000291514. [DOI] [PubMed] [Google Scholar]
- 75.Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr. Res. 2012;137:246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fervaha G, Agid O, Foussias G, Remington G. Toward a more parsimonious assessment of neurocognition in schizophrenia: a 10-minute assessment tool. J. Psychiatr. Res. 2014;52:50–56. doi: 10.1016/j.jpsychires.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Nuechterlein KH, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 78.Fervaha G, et al. Examination of the validity of the Brief Neurocognitive Assessment (BNA) for schizophrenia. Schizophr. Res. 2015;166:304–309. doi: 10.1016/j.schres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 79.Crowe SF. Does the letter number sequencing task measure anything more than digit span? Assessment. 2000;7:113–117. doi: 10.1177/107319110000700202. [DOI] [PubMed] [Google Scholar]
- 80.Juckel G, et al. Validation of the Personal and Social Performance (PSP) Scale in a German sample of acutely ill patients with schizophrenia. Schizophrenia Res. 2008;104:287–293. doi: 10.1016/j.schres.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 81.Gershman SJ, Tzovaras BG. Dopaminergic genes are associated with both directed and random exploration. Neuropsychologia. 2018;120:97–104. doi: 10.1016/j.neuropsychologia.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Somerville LH, et al. Charting the expansion of strategic exploratory behavior during adolescence. J. Exp. Psychol. Gen. 2017;146:155–164. doi: 10.1037/xge0000250. [DOI] [PubMed] [Google Scholar]
- 83.Assarsson E, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PloS ONE. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- 85.RCoreTeam. R: A Language and Environment for Statistical Computing [Internet]. R Foundation for Statistical Computinghttps://www.R-project.org (2017).
- 86.Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav. Res. Methods. 2018;50:195–212. doi: 10.3758/s13428-017-0862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991;21:1129–1164. doi: 10.1002/spe.4380211102. [DOI] [Google Scholar]
- 88.Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: generalizing degree and shortest paths. Soc. Netw. 2010;32:245–251. doi: 10.1016/j.socnet.2010.03.006. [DOI] [Google Scholar]
- 89.Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: network visualizations of relationships in psychometric data. J. Stat. Softw. 2012;48:18. doi: 10.18637/jss.v048.i04. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.