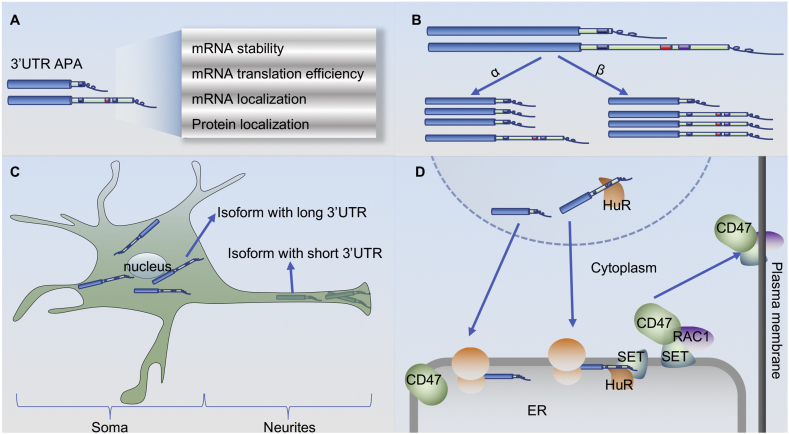
Figure 4.
Impact of 3′UTR APA on its target mRNA and protein. (A) Summary of potential consequences of 3′UTR alternative cleavage and polyadenylation on the affected mRNA and its encoded protein. (B) Long 3′UTRs contain more cis elements than short 3′UTRs, influencing both the stability and translation rate of the isoforms. The α pathway depicts the effect of cis elements such as miRNA binding sites in the long 3′UTR, promoting degradation of the lengthened isoform. The β pathway depicts the effect of 3′UTR shortening in increasing the miRNA binding efficiency in the middle region of the 3′UTR, thus decreasing the stability of the shortened isoform. In addition, some cis elements in the 3′UTR contribute to translation efficiency differences between transcripts bearing a longer or shorter 3′UTR. (C) Transcripts that undergo APA at the distal PAS remain in the soma of neurons, whereas those that use proximal PAS preferentially localize to neurites. (D) Cis-regulatory elements in 3′UTRs can direct the cellular localization of the encoded protein by altering interactions with RNA-binding proteins. The 3′UTR of the long isoform of CD47 can be bound by HuR and recruits SET to the translation site, facilitating the nascent CD47 protein to join active RAC1 and translocate to the plasma membrane.