Abstract
Objective
Pediatric cancer patients endure multiple symptoms during treatment and also in survivorship. Digital health technologies provide an innovative way to support their symptom management. This review aimed to examine the effect of digital health technologies on managing symptoms among across pediatric cancer continuum.
Methods
A systematic literature search of six English and three Chinese electronic databases was combined with hand searching, to identify eligible research studies from database establishment to November 30, 2019. Two reviewers carried out data selection, data extraction, and quality appraisal independently. A narrative approach was taken to summarize data.
Results
Four randomized control trials, two quasi-experiments, and five one group pre-posttest designed studies, were included in the review with a total of 425 participants. The methodological quality of the studies was generally fair. Seven symptoms (anxiety, depression, pain, anger, fatigue, fear, distress) and seven digital health technologies (visual reality, website, humanoid robot, app, wearable devices, short messages and videoconference) were reported in the included studies.
Conclusions
Current evidence supports the effect of digital health technologies is generally mixed and inconclusive. There is a trend of positive effects found in the interventions that feature digital health technologies’ interactive function. This review highlights the need for further investigation with rigorous research designs and the consideration of influencing factors from the symptoms, participants, and context levels to inform a better digital health implementation.
Keywords: Child, Neoplasms, Palliative care, Symptom assessment, Telemedicine, Wearable electronic devices
What is known?
-
•
Pediatric cancer patients endure multiple symptoms during treatment and also in survivorship. Recent evidence has demonstrated the feasibility and acceptability of some digital health interventions.
What is new?
-
•
Current evidence supports the effect of digital health technologies is generally mixed and inconclusive. There is a trend of positive effects found in the interventions that feature digital health technologies’ interactive function.
-
•
When examining the effect, there is a need to take into consideration about the factors from the symptoms, participants, and context levels.
1. Introduction
Each year, approximately 300,000 children were diagnosed with cancer worldwide [1]. The advancement of treatments for childhood cancers has significantly improved the survival rate. However, these aggressive treatments for childhood cancers have also induced symptom burdens and suffering for pediatric cancer patients. Studies has proved that pediatric cancer patients can report up to 15 distressing and disruptive symptoms, with anxiety, depression, pain, and fatigue the most frequently cited [2,3]. These multiple interrelated occurring symptoms directly affect patients’ ability to function in multiple domains and their overall quality of life. It is noted that some of the symptoms may prolong to survivorship. For instance, fatigue is not only a distressing symptom frequently reported by children undergoing cancer treatment; but also is one of the most common complaints of survivors of childhood cancer [4]. In the meantime, those childhood cancer survivors are also faced with an increased risk of late effects from treatment (e.g. accumulated toxicity from previous chemotherapy), along with cancer-or-treatment related long-term multisystem health conditions [5]. Moreover, for pediatric cancer patients who grow into adolescents or young adults, immerging autonomy may influence their health behaviors, which may bring new physical or psychological symptoms that impact the quality of life [6]. In light of the disease, treatment and developmental influencing factors discussed above, it will be difficult to separate the multiple impacts and complex healthcare needs across the pediatric cancer continuum [7]. Evidence on managing symptoms across the pediatric cancer continuum is therefore warranted.
Among all the symptom management interventions, digital health technology has become an emerging hot topic. Digital health technology is broadly defined as the use of technology in the promotion, prevention, treatment, and maintenance of health and health care [8]. It includes mobile health (mHealth), health information technology (IT), text messaging, apps, wearable devices, telehealth and telemedicine, digital gaming, virtual reality, robotics, online support groups and social networks [[8], [9], [10]]. In the past decade, digital health technologies have risen in prominence [10,11] and become a promising and innovative facilitator that expands the scope of health care [12,13]. Substantial evidence has proved that digital health technologies can overcome the barriers of geographical and time-related constraints and exemplified their strength in various ways, e.g., assisting health information delivery, patient-clinician communications, social networking, real-time health indicator monitoring, and automated clinical intervention [14,15].
Regarded as “digital native”, the young generations are exposed every day to a technology-driven environment with digital devices [16]. Age-appropriate co-design and cognitive interviews have been widely used to develop digital health technologies for children of all ages [17,18]. In the field of pediatric oncology, digital health technology is pointed out as one of the promising strategies that support patients and survivors to manage the challenges associated with their disease and treatment [15]. Currently, synthesized evidence has demonstrated the feasibility and acceptability of digital health interventions on this particular population [8,10]. McCann’s systematic review of 38 studies assessed the quality, feasibility, and efficacy of existing digital health interventions that support adolescents and young adults with cancer. The authors summarized the function of most frequently used healthcare technologies and pointed out that symptom management was a major focus. However, they concluded that the technologies “have yet to be evaluated at scale”. In another systematic review led by Ramsey, the authors appraised the current scientific evidence on electronic health (eHealth) and mobile health (mHealth) interventions in study participants from child to young adult. The authors concluded from the twenty-one studies met the inclusion criteria that although feasibility and acceptability were established for digital health technologies. Evidence of efficacy for interventions targeting emotional distress, health behaviors, health outcomes, and neurocognitive functioning was still mixed. Both of the systematic reviews mentioned the important role of digital health technologies in symptom management. However, neither of them further analyzed the evidence from the symptom perspectives. There is a lack of synthesized evidence specifically on the effect of digital health technologies on managing symptoms across the continuum of childhood cancer treatment and survivorship. This gap will prevent the understanding of symptom management function and further develop digital health intervention strategies that support these pediatric cancer patients and survivors to cope with their symptoms.
2. Methods
The purpose of this review was to examine the effect of digital health technologies on managing symptoms among across pediatric cancer continuum. This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendation [19].
2.1. Eligibility criteria
Participants included children, adolescents and young adults with cancer (0–39 years old), and young adult survivors of childhood cancer were defined as mean age 21 years or younger at the time of diagnosis; mean age 39 years or younger at the time of intervention [11].
The intervention incorporated using digital health technology as the component. In this study, “digital health technology” included but not limited to mobile health (mHealth), health information technology (IT), text messaging, apps, wearable devices, telehealth and telemedicine, digital gaming, virtual reality, robotics, online support groups and social networks [[8], [9], [10]]. The comparator comprised of studies in which the comparison group did not receive intervention from using digital health technology.
The studies must include at least one outcome related to the patients’ symptoms. “Symptoms” were defined as clinical manifestations that can be either objective when observed by a physician, or subjective when perceived by the pediatric cancer patients or survivors.
The designs included randomized controlled trials (RCTs), quasi-experimental studies, pre-posttests, interrupted time series designed studies.
Exclusion criteria are studies that 1) had insufficient detail on the target population, 2) had a vague description of the digital health technology of intervention, and 3) did not examine patients’ symptoms.
2.2. Literature search
The first two authors performed a thorough database searching in six English databases (Cochrane Library, Joanna Briggs Institute Library, PubMed, CINAHL, EMBASE, and Web of Science) and three Chinese databases (Wanfang Database, National Knowledge Infrastructure (CNKI) and SinoMed) from the databases’ inception to November 30, 2019. Additional records were identified through hand searching. A combination of both keywords and indexed terms (e.g., MeSH) was applied in each database using BOOLEAN terms. Search strategies for each database were listed in Appendix A. This literature search was conducted from December 15, 2019 to July 1, 2020.
2.3. Study selection
Two reviewers independently identified the titles and abstracts of studies by the search strategies. Potentially eligible studies were evaluated at full text according to the inclusion and exclusion criteria, and the final inclusion of studies into the systematic review was by agreement of all the authors.
2.4. Data extraction
Data were extracted with a report table, including first author, year, country, study design, sample, symptom and measurement, intervention, and symptom(s) related outcomes (Table 1). Two reviewers independently verified all of the extracted content.
Table 1.
A summary of research studies included in the review.
| Author, year and country | Design | Sample | Symptom (measurement) | Intervention | Symptom(s) related outcomes |
|---|---|---|---|---|---|
| Alemi et al., 2016 [24] Iran |
Quasi-experiment, 8-week, pre-post test | 11 pediatric cancer patients (aged 7–12), cancer types not reported, from two hospitals. Experiment group (n = 6): social robot-assisted therapy; Control group (n = 5): psychotherapy. |
Reported by patients Anxiety(Multidimensional Anxiety Children Scale) Depression(Children’s Depression Inventory) Anger(Children’s Inventory of Anger) |
Social humanoid robot-assisted therapy: 8 scenarios | Experimental group experienced greater reductions in anxiety, depression and anger than control group (all P < 0.05). |
| Campo et al., 2017 [26] USA |
One group, pre-post test | 25 young adult cancer survivors (aged 18–29), all types of cancer, 2.6 years since treatment completion | Reported by patients Anxiety(PROMIS--Anxiety v1.0 short-form) Depression(PROMIS--Depression v1.0 short-form) |
Telehealth: mindful self-compassion videoconference intervention, group-based, 90-min videoconference sessions, held weekly over 8 weeks, with audio-supplemented home practice | Anxiety and depression level demonstrated significant reduction (P < 0.001), with large effect sizes (Cohen’s d 1.24, 0.99, separately). |
| Gershon et al., 2004 [20] USA |
RCT, three arms parallel, 8-week, pre-post test | 59 pediatric cancer patients (aged 7–19), with all types of cancer, need port access, from at an outpatient oncology. Experiment group (n = 22): Virtual Reality; Distraction group (n = 22): non–Virtual Reality distraction; Control group (n = 15): usual care. |
Reported by patients, parents, nurses Pain(VAS) Anxiety(VAS) |
eHealth: Virtual Reality, immersive Virtual Reality distraction technique | Only nurse reported pain for the experimental and distraction groups were significantly reduced, compared to the control group at posttest (P < 0.05). Younger children experienced more distress with this procedure than older children. |
| Hooke et al., 2016 [27] USA |
One group, three time points: baseline, after 2 weeks (i.e. before the steroid pulse), and after 5 days of steroids | 17 pediatric patients (aged 6–18) with ALL receiving a cycle of maintenance chemotherapy that included full doses of a corticosteroid (dexamethasone or prednisone) | Reported by patients Fatigue(Childhood Fatigue Scale for children ages 6–12; Fatigue Scale for Adolescents in adolescents 13–18 years) |
Wearable technology: Fitbit tracker as a pedometer-based intervention with daily coaching for 2 weeks before a maintenance steroid pulse | No significant differences observed for fatigue at posttest (P > 0.05). Higher steps were associated with lower fatigue (r = −0.563, P = 0.029) |
| Huang et al., 2014 [21] USA |
RCT, two arms parallel, 4-month, pre-post, stratified by age | 38 pediatric cancer survivors with ALL (aged 8–18) with BMI≥85%. Experiment group (n = 19): Fit4Life; Control group (n = 19): printed weight management materials. |
Reported by patients Depression(Children’s Depression Inventory) |
mHealth: a WMI tailored for childhood ALL survivors (Fit4Life), 4-month web, phone, and text message-delivered WMI tailored for cancer survivorship | Experimental group reported reduced depression compared to control at posttest (P = 0.02). |
| Jibb et al., 2017 [28] Canada |
One group, pre-post test | 40 pediatric cancer patients (aged 12–18) with all types of cancer undergoing cancer treatment | Reported by patients Pain intensity(Brief Pain Inventory) Pain interference(PROMIS Pediatric Pain Interference Short-form Scale) |
mHealth: Pain Squad + APP, electronic monitoring with real-time self-management recommendations | Change scores showed each pain intensity item improved over the course of Pain Squad + use (All P < 0.05). Less pain interference post intervention as compared to baseline with a small effect size (Cohen’s d = 0.38; P = 0.039). |
| Kunin-Batson et al., 2016 [22] US |
RCT, two arms parallel, 12-month, pre-post test | 52 AYA cancer survivors (aged 15–29) with all types of cancer. Experiment group (n = 26): web-based information provision; Control group (n = 26): standard of care. |
Reported by patients Anxiety(State Trait Anxiety Inventory) |
eHealth: receive access to personalized health history, late effects information, and resources via a password-protected web portal. | No significant differences on anxiety between groups (P > 0.05). |
| Li et al., 2011 [25] China |
Quasi-experiment, two arms, pre-post test | 122 pediatric cancer patients (aged 8–18) with all types in treatment. Experiment group (n = 52): therapeutic play; Control group (n = 70): routine nursing care. |
Reported by patients Anxiety(Chinese Version of the State Anxiety Scale for Children) Depressive symptoms(Center for Epidemiologic Studies Depression Scale for Children) |
eHealth: therapeutic play, using Virtual Reality computer games: conducted by research nurse and implemented in small group with maximum four children in one group in a playroom of the oncology unit. | Experimental group reported statistically significant fewer depressive symptoms than children in the control group on day 7 (P = 0.02). No differences in children’s anxiety scores between the two groups on day 7 (P = 0.07). |
| Sander et al., 2002 [23] USA |
RCT, two arms post test | 30 adolescents (aged 10–19), with all types in treatment during lumbar punctures. Experiment group (n = 17): Virtual Reality; Control group (n = 13): standard care. |
Reported by patients Pain(VAS) |
eHealth: Virtual Reality, wore Virtual Reality glasses and watched a video | Pain scores were not statistically different between the two groups (P = 0.77). Pain scores tended to be lower in the Virtual Reality group (median score of 7.0, range 0–48) than in the control group (median score of 9.0, range 0–59). |
| Schneider et al., 1999 [29] USA |
An interrupted time series design, one group | 11 children (aged 10–17) with all types of cancer receiving chemotherapy. | Reported by patients Symptom distress(Symptom Distress Scale) Anxiety(State-Trait Anxiety Inventory for Children) |
eHealth: Virtual Reality, wore a Virtual headset with one of three CD ROM-based scenarios, during a single intravenous chemotherapy treatment | Symptom distress score during the initial chemotherapy treatment decreased during subsequent treatments (P = 0.02). State anxiety levels were not influenced by the Virtual Reality intervention (P = 0.11). |
| Seitz et al., 2014 [30] Germany |
One group, 3-month, pre-post test | 20 cancer survivors (aged 20–36) with all types of cancer. | Reported by patients Anxiety, depression(Hospital Anxiety and Depression Scale) Fear(Short form of the Fear of Progression and Relapse Questionnaire) |
eHealth: web based therapist guided, cognitive behavioral intervention: 10 writing sessions containing standardized text messages and instructions | Significant decreases in anxiety (t = 3.44; P = 0.003), fear of relapse/progression (t = 2.14; P = 0.046), and depression (t = 5.69; P < 0.001). |
Note: ALL = acute lymphoblastic leukemia. AYA = adolescent and young adult. PROMIS = Patient-Reported Outcomes Measurement Information System. VAS = Visual Analog Scale. WMI = weight management intervention.
2.5. Quality assessment of included studies
The first two authors assessed the included studies’ quality independently according to a 27-item Checklist for Both Randomized and Nonrandomized Studies [19]. This checklist includes five subscales: reporting, external validity, bias, internal validity, and statistical power. The maximum total score in this assessment was 28. Studies scoring 9 or less (<33%) were considered of low quality.
2.6. Data analysis
A narrative synthesis was performed given the heterogeneous nature of the interventions involved and the outcome measures investigated across selected studies. For each symptom, its relevant digital health technology interventions, mode of delivery, and effects were also summarized.
3. Results
3.1. Study characteristics
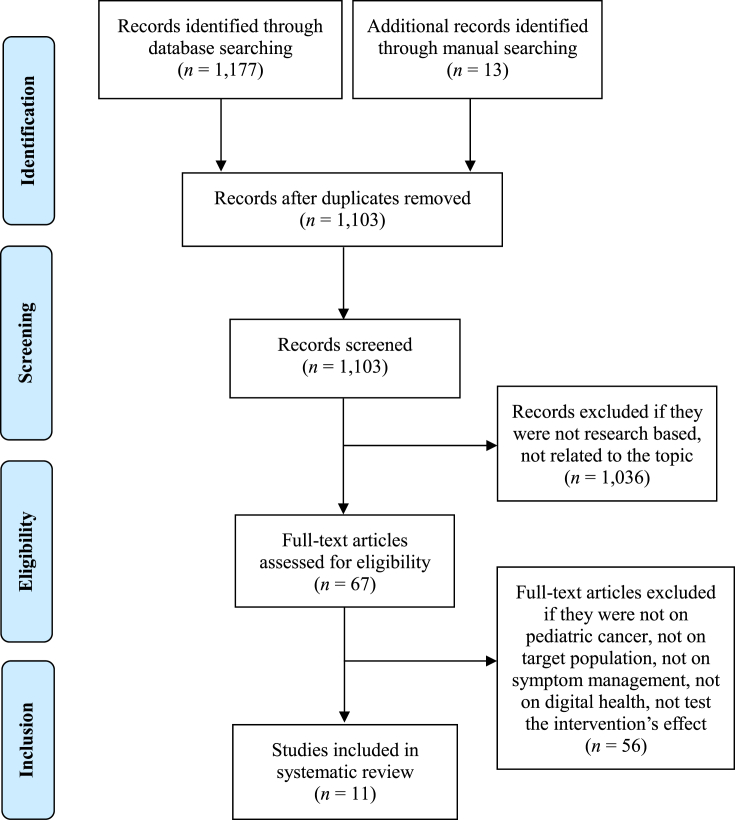
The search strategy identified a total of 1,190 articles. After removing duplicates, the remaining 1,103 articles were screened by titles and abstracts, resulting in 67 articles remaining. Of the retrieved full-text publications, 56 were excluded, leaving 11 papers included in this review. Fig. 1 illustrates the flow diagram of studies identification and selection. Table 1 shows the characteristics of the included publications. Appendix B displays all the studies that were excluded from the full-text assessment and why each was excluded.
Fig. 1.
Prisma flow diagram.
Most of the studies were conducted in the United States (n = 8), one in Canada, one in Germany, and one in China. Of the eleven included studies, four were randomized control trials [[20], [21], [22], [23]], two quasi-experiment designs [24,25], and five one group pre-posttests [[26], [27], [28], [29], [30]]. The time intervals for measurement varied from 1 week to 12 months.
In terms of the participants’ characteristics, their ages, diagnosis and treatment stages varied across the studies. Specifically, our studies included both children and adolescents (6–20 years old)[ 20,21, 24, 25, 27], three studies enrolled only adolescents (10–20 years old)[ 23, 28, 29], two studies recruited both adolescents and young adults (15–39 years old)[ 22, 26], the rest one study only included young adults (18–35 years old) [30]. Most studies had participants with all types of cancer, except for two studies recruited children with acute lymphoblastic leukemia [21,27]. Five studies recruited children undergoing active treatment [[23], [24], [25], [26],29]. The other six studies focused on participants in survivorship [[20], [21], [22],27,28,30].
3.2. Symptoms used as outcome indicator(s)
Symptom(s) were used as the primary outcome indicator(s) in ten studies, and secondary outcome in one study [21]. Among the symptoms, anxiety was the main indicator (n = 7), following by depression (n = 4), pain (n = 3), anger (n = 1), fatigue (n = 1), fear (n = 1), and general symptom distress (n = 1). Most studies (n = 10) collected participants’ self-reported symptoms with age-appropriate instruments. One study measured different symptom reports by children, parents, and nurses [20].
3.3. Digital health technology-assisted intervention
The digital health technology-assisted interventions mainly incorporated visual reality, website, humanoid robot, app, wearable devices, short messages and videoconference. Specifically, four studies used visual reality as a distraction to reduce the anxiety and pain associated with invasive port device access procedures [20], minimize the anxiety and depressive symptoms during hospitalization [25], alleviate the pain during a lumbar puncture [23], and mitigate chemotherapy-related symptom distress [29]. Three studies developed their interventions online, which respectively incorporated a mindful self-compassion videoconference for anxiety and depression alleviation [26], a web-based resource with individually tailored information [22], an internet-based cognitive-behavioral intervention [30]. For the rest four studies, one study used a social humanoid robot as a therapy-assistive tool in dealing with pediatric distress [24]. Another study developed a Pain Squad + smartphone app to alleviate pain [28]. The third study employed a fitness tracker as wearable technology to promote physical activity and diminish fatigue [27]. One study combined web, phone, and text messages to deliver a tailored weight management intervention to relieve the negative mood in cancer survivorship [21].
3.4. Effect of interventions on managing symptoms (Table 2)
Table 2.
Symptoms and effects of digital health technology.
| Symptoms | Digital health intervention delivery | Effect |
|---|---|---|
| Anxiety | Robotic [24] | + |
| Visual Reality [20] | – | |
| Visual Reality [25] | – | |
| Visual Reality [29] | – | |
| Videoconference [26] | + | |
| Web-based information provision [22] | – | |
| Web based therapist guided, cognitive behavioral intervention [30] | + | |
| Depression | Robotic [24] | + |
| Videoconference [26] | + | |
| Web, phone, and text message-delivery [21] | + | |
| Visual Reality [25] | + | |
| Pain | Visual Reality [20] | +/− |
| Visual Reality [23] | – | |
| APP [28] | + | |
| Anger | Robotic [24] | + |
| Fatigue | Wearable technology [27] | – |
| Fear | Web based therapist guided, cognitive behavioral intervention [30] | + |
| Symptom distress | Visual Reality [29] | – |
Note: + positive results; - negative results; +/− mixed results.
Seven studies investigated the effect of digital health technology on anxiety. The interventions involved included visual reality [20,25,29], web-based technology [22,26,30], and a humanoid robot [24]. Alemi et al. found a significant between-group difference in reductions in anxiety (P < 0.05) for children who received social humanoid robot-assisted therapy [24]. However, none of the three studies on visual reality using detected a significant positive effect on reducing anxiety, either by visual reality virtual reality immersive distraction technique [20], or virtual reality computer games [25]or virtual reality scenarios [29]. Meanwhile, the three studies involving online interventions released mixed results. Two studies reported a significant reduction in anxiety (Cohen’s d = 1.24, P < 0.001 and t = 3.44, P = 0.003, respectively) [26,30]. A third study did not find any significant difference (P > 0.05) [22].
Four studies reported the effect of the interventions on depression. The involved interventions were a humanoid robot [24], web-based technology [26], visual reality [25], and a combined web, phone, and text messages delivery [21]. Their results were consistently positive. In Alemi’s study, children expressed less depression when receiving the social humanoid robot-assisted therapy significantly (M = 0.012, F (1, 8) = 8.66, P < 0.05) [24]. Campo’s study suggested a significant decrease in depression level after a mindful self-compassion videoconference intervention (Cohen’s d 0.99, P < 0.001) [26]. In the virtual reality computer games led by Li, children in the experimental group reported fewer depressive symptoms compared to those in the control group (P = 0.02) [25]. Likewise, in Huang’s study, depression was reduced in participants receiving web, phone, and text message delivery (P = 0.02) [21].
Three studies investigated the effect of digital health technology interventions on pain. The interventions involved included visual reality [20,23] and a smartphone app [28]. The results were inconsistent. Neither of the two studies on visual reality interventions detected a statistical difference in patient-reported VAS pain scores between the experiment and control groups. However, a significant posttest decrease in nurse-reported pain scores was noted in one of two studies [20]. A Pain Squad + APP was used in Jibb’s study, which revealed a significantly lower pain intensity (P < 0.05) and less pain interference post-intervention (Cohen’s d = 0.38, P = 0.039) [28].
One study assessed the effect of humanoid robot interventions on anger. In Alemi 2016’s study, social humanoid robot-assisted therapy demonstrated its strength in releasing the participants’ anger level (M = 0.216, F (1, 8) = 10.28, P < 0.05).
One study reported the effect of wearable technology interventions on fatigue. In the study led by Hooke, Fitbit tracker was used as a pedometer-based intervention with daily coaching for children with acute lymphoblastic leukemia prior to a maintenance steroid pulse. Although higher steps were associated with lower fatigue (r = −0.563, P = 0.029), no significant differences were noted in fatigue at a two-week posttest (P = 0.42) [27].
One study assessed the effect of web-based therapist guided, cognitive-behavioral intervention on fear. In Seitz’s study, significant decreases in fear of relapse/progression in the 3-month post-test (t = 2.14, P = 0.046) [30].
One study reported the effect of virtual reality interventions on symptom distress. In Schneider’s study, the participants’ symptom distress score decreased during subsequent treatments compared to the initial chemotherapy treatment (P = 0.02) [29].
3.5. Level of methodological quality
The studies’ quality ranged from moderate to high, with overall scores ranging between 16 and 21 out of 28. All the 11 articles attained a score of 8 or above for the reporting subscale. None of them explicitly described the occurrence of adverse events. The external validity score of the 11 studies was generally fair, from 1 to 3 out of 3. All studies achieved 5 out of 7 for the bias subscale, as blinding was not feasible considering the intervention’s nature. The studies achieved a medium score (2–4 out of 6) on the internal validity subscale, as it was not feasible for assignment concealment. Power analysis was only carried out in 2 studies for sample size estimation (Table 3).
Table 3.
Quality of studies.
| Author and year | Reporting (Max = 11) | External validity (Max = 3) | Bias (Max = 7) | Internal validity (Max = 6) | Power (Max = 1) | Total score |
|---|---|---|---|---|---|---|
| Alemi et al., 2016 [24] | 8 | 1 | 5 | 3 | 0 | 17 |
| Campo et al., 2017 [26] | 9 | 3 | 5 | 2 | 0 | 19 |
| Gershon et al., 2004 [20] | 8 | 1 | 5 | 4 | 0 | 18 |
| Hooke et al., 2016 [27] | 8 | 1 | 5 | 2 | 0 | 16 |
| Huang et al., 2014 [21] | 9 | 3 | 5 | 4 | 0 | 21 |
| Jibb et al., 2017 [28] | 8 | 1 | 5 | 3 | 0 | 17 |
| Kunin-Batson et al., 2016 [22] | 9 | 3 | 5 | 4 | 0 | 21 |
| Li et al., 2011 [25] | 9 | 1 | 5 | 2 | 1 | 18 |
| Sander et al., 2002 [23] | 9 | 1 | 5 | 4 | 0 | 19 |
| Schneider et al., 1999 [29] | 9 | 1 | 5 | 2 | 1 | 18 |
| Seitz et al., 2014 [30] | 9 | 1 | 5 | 2 | 0 | 17 |
4. Discussion
In this review, we examined the effect of digital health technologies on managing symptoms across the pediatric cancer continuum. There were seven digital health technologies aimed at addressing seven symptoms in pediatric cancer patients and survivors. Our findings were generally consistent with previous reviews that current evidence supports this intervention is generally mixed and inconclusive [31]. Of note, there is a trend that more positive effects were found in the interventions that feature the interactive function of digital health technologies. Meanwhile, influencing factors from the symptoms, participants, and context may influence the intervention’s effects.
In this review, the main categories of digital health technologies included the internet (web-based information provision, web-based therapy), mobile health (app, text messages and wearable devices), telehealth (videoconference), and emerging technologies (visual reality and humanoid robot). The functions of these technologies varied from information deliveries to interactive communications. In general, the technology that involved more interactive features (e.g., web-based therapy, apps, videoconference, humanoid robot) was likely to generate positive evidence that supports the symptom management across the pediatric cancer continuum. For instance, the internet assists in delivering high-quality and tailored symptom management information [32]. However, in this review, only when used in conjunction with interactive components (e.g., web-based therapy, phone, and text message) could the benefit of the internet be evident [21,30]. The simple application of online information provision did not lead to positive outcomes [22]. Of the three mobile health and one telehealth technologies, their common strengths were consistently shown in symptom management regarding real-time data collection and response, user convenience, patient-clinician connections and interactions. Likewise, in terms of the two emerging digital health technologies, while interactive humanoid robots were proved to be capable to alleviate symptoms, there remained mixed outcomes from the studies on distractions by virtual reality. Obviously, the importance of an interactive feature of digital health technology should not be overlooked in its development and implementation stages. Particularly, for young children with cancer, this feature will catch their attention and get them involved. While for adolescents and young adults with cancer, a digital intervention with an interactive feature will give them a feeling of being recognized and cared for, which is essential for their development of autonomy and self-conscience in symptom management.
When examining the effect of digital health technology, there is a need to take into consideration about the influencing factors from the symptoms, participants, and context levels. First, with respect to symptoms, compared to the various symptoms experienced by children and adolescents with cancer [3], this review only found a limited number of symptoms intervened by digital health technologies. Of note, most of the symptoms were related to psychological health. Although it is widely reported that intersecting and troubling symptoms (e.g., fatigue, pain and depression) often coexist and are recognized as a part of a symptom cluster [33,34] through a common underlying pathophysiological mechanism, e.g., systemic inflammation [35]. Few studies estimate the synergy effects of symptoms or leverage their digital health interventions according to the targeted symptoms’ relationships.
Second, the characteristics of participants may influence the usability and implementation of digital health technologies. For pediatric cancer patients and survivors, their developmental stages and malignancy treatment are twofold factors that interfere with the intervention. Specifically, the developmental issue needs to be considered when designing the digital health interventions’ layout and function, impacting participants’ preference and involvement. As exemplified in this review, only two studies applied technologies targeting a particular age group, i.e., social humanoid robot-assisted therapy for children aged 6–12 years, and web-based therapy for young adults. Meanwhile, the influence of participants’ disease and treatment should not be overlooked, e.g., neuropsychological complications may prevent a participant from using specific technologies like digital cognitive therapy. Furthermore, it is unlikely to underestimate the impact of other co-existing non-technological symptom interventions, e.g., the simultaneous usage of EMLA cream for pain alleviation during lumbar puncture [23], other forms of off-line health education (paper format materials, booklets, etc.), and in person symptom management interventions.
Third, the contextual factors that influence digital health technologies’ effect may come from users’ environment, resources, and culture. It is noted that the majority of the included studies were from developed countries. Participants’ financial and technological capabilities may shape their participation willingness. However, there was limited information on how socioeconomic influencers (e.g., digital resources, sex, family income, or parent education) affects intervention outcomes. Likewise, there is limited information on any direct or indirect costs and health outcomes of different interventions. In light of the variations of health care models and payment methods, it was impossible to perform a cost-effective analysis that examines the interventions’ effect from a health economic lens. Meanwhile, cultural factors were rarely mentioned in the included studies. It would be worthwhile to explore how certain cultural elements (e.g., the tolerance level of self-exposure during videoconference) impact or moderate the effect of digital health technologies.
This systematic review has limitations. First, the search strategies of this review only involved major databases without grey literature. There is a possibility that some literature may be missed. Second, the studies included in this review were a mixture of randomized controlled trials, pilot studies, or pre-posttests on feasibility and efficacy. The methodological limitations (e.g., small sample size and lack of control groups) prevented the synthesis and evaluation of the strength of overall evidence. Third, there was considerable heterogeneity in the age group, patients’ developmental level, and sample size of each included study. It was not practical to perform meta-analysis or any sub-analysis but a narrative data synthesis.
Future studies on the effect of digital health technology on symptom management are recommended to address the following areas. First, large scale, robustly designed studies are demanded to test digital health interventions on this particular population. Extended follow-up is especially needed to test the effect alongside the point of diagnosis, during treatment, and in survivorship. Second, there is paucity in current research to explore the relationships between digital health implementation within routine care provision. Particularly, how the enabled distance and real-time symptom management technologies supplement face-to-face delivery of care for those who are restrained from trained providers due to distance, mobility difficulties, and cancer stigma. Third, it is necessary to fully interpret the effect of digital health technology on symptom management across the cancer continuum with consideration of the multiple influencing factors from symptoms, participants, and context levels.
5. Conclusion
At present, digital health technology serves an increasingly important role in managing symptoms across pediatric cancer continuing. However, this review suggested that current evidence supports the effect of this intervention is generally mixed and inconclusive. Of note, there is a trend of positive effects found in the interventions that feature interactive function. This review highlights the need for further investigation with rigorous research designs and increased sample sizes to further examine the effect alongside the pediatric cancer continuum. Meanwhile, influencing factors from the symptoms, participants, and context need to be considered to inform a better digital health implementation.
CRediT authorship contribution statement
Lei Cheng: Conceptualization, Methodology, Validation, Writing - original draft, Writing - review & editing. Mingxia Duan: Investigation, Data curation, Writing - review & editing. Xiaorong Mao: Investigation, Data curation, Writing - review & editing. Youhong Ge: Investigation, Data curation, Writing - review & editing. Yanqing Wang: Investigation, Data curation, Writing - review & editing. Haiying Huang: Data curation, Writing - review & editing.
Statement of ethics approval
This study does not need ethical approval as it is a systematic review of literature.
Funding
This study was supported by the China National Natural Science Foundation of China Youth Science Foundation (71904030), and Shanghai Pujiang Talent Program (2019PJC006).
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2020.10.002.
Appendices. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Who . 2018. Global initiative for childhood cancer.https://www.who.int/cancer/childhood-cancer/en/ Published 2018. [Google Scholar]
- 2.Wolfe J., Orellana L., Ullrich C., Cook E.F., Kang T.I., Rosenberg A. Symptoms and distress in children with advanced cancer: prospective patient-reported outcomes from the PediQUEST study. J Clin Oncol. 2015;33(17):1928–1935. doi: 10.1200/jco.2014.59.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng L., Wang L., He M., Feng S., Zhu Y., Rodgers C. Perspectives of children, family caregivers, and health professionals about pediatric oncology symptoms: a systematic review. Support Care Canc. 2018;26(9):2957–2971. doi: 10.1007/s00520-018-4257-3. [DOI] [PubMed] [Google Scholar]
- 4.Karimi M., Cox A.D., White S.V., Karlson C.W. Fatigue, physical and functional mobility, and obesity in pediatric cancer survivors. Canc Nurs. 2020;43(4):E239–E245. doi: 10.1097/ncc.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 5.Langer T., Grabow D., Kaatsch P., Creutzig U., Eggert A., Escherich G. Long-Term Follow-Up in Childhood Cancer Survivors - position paper 2018 of the working group "long-term follow-up" of the Society of Pediatric Oncology and Hematology (GPOH) on long-term surveillance, long-term follow-up and late effect evaluation in pediatric oncology patients. Klin Pädiatr. 2018;230(6):291–298. doi: 10.1055/a-0754-2362. [DOI] [PubMed] [Google Scholar]
- 6.Zebrack B., Mathews-Bradshaw B., Siegel S. Quality cancer care for adolescents and young adults: a position statement. J Clin Oncol. 2010;28(32):4862–4867. doi: 10.1200/jco.2010.30.5417. [DOI] [PubMed] [Google Scholar]
- 7.Fern L.A., Taylor R.M., Whelan J., Pearce S., Grew T., Brooman K. The art of age-appropriate care: reflecting on a conceptual model of the cancer experience for teenagers and young adults. Canc Nurs. 2013;36(5):E27–E38. doi: 10.1097/NCC.0b013e318288d3ce. [DOI] [PubMed] [Google Scholar]
- 8.Devine K.A., Viola A.S., Coups E.J., Wu Y.P. Digital health interventions for adolescent and young adult cancer survivors. JCO Clin Cancer Inform. 2018;2:1–15. doi: 10.1200/cci.17.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrelli B., Ritterband L.M. Special issue on eHealth and mHealth: challenges and future directions for assessment, treatment, and dissemination. Health Psychol. 2015;34s:1205–1208. doi: 10.1037/hea0000323. [DOI] [PubMed] [Google Scholar]
- 10.McCann L., McMillan K.A., Pugh G. Digital interventions to support adolescents and young adults with cancer: systematic review. JMIR Cancer. 2019;5(2) doi: 10.2196/12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey W.A., Heidelberg R.E., Gilbert A.M., Heneghan M.B., Badawy S.M., Alberts N.M. eHealth and mHealth interventions in pediatric cancer: a systematic review of interventions across the cancer continuum. Psycho Oncol. 2019 doi: 10.1002/pon.5280. [DOI] [PubMed] [Google Scholar]
- 12.Oh H., Rizo C., Enkin M., Jadad A. What is eHealth (3): a systematic review of published definitions. J Med Internet Res. 2005;7(1):e1. doi: 10.2196/jmir.7.1.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesley K.M., Fizur P.J. A review of mobile applications to help adolescent and young adult cancer patients. Adolesc Health Med Therapeut. 2015;6:141–148. doi: 10.2147/ahmt.s69209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts A.L., Fisher A., Smith L., Heinrich M., Potts H.W.W. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2017;11(6):704–719. doi: 10.1007/s11764-017-0632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris J., Cheevers K., Armes J. The emerging role of digital health in monitoring and supporting people living with cancer and the consequences of its treatments. Curr Opin Support Palliat Care. 2018;12(3):268–275. doi: 10.1097/spc.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 16.Pathipati A.S., Azad T.D., Jethwani K. Telemedical education: training digital natives in telemedicine. J Med Internet Res. 2016;18(7):e193. doi: 10.2196/jmir.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bird M., Li L., Ouellette C., Hopkins K., McGillion M.H., Carter N. Use of synchronous digital health technologies for the care of children with special health care needs and their families: scoping review. JMIR Pediatr Parent. 2019;2(2) doi: 10.2196/15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bevan Jones R., Stallard P., Agha S.S., Rice S., Werner-Seidler A., Stasiak K. Practitioner review: Co-design of digital mental health technologies with children and young people. JCPP (J Child Psychol Psychiatry) 2020 doi: 10.1111/jcpp.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershon J., Zimand E., Pickering M., Rothbaum B.O., Hodges L. A pilot and feasibility study of virtual reality as a distraction for children with cancer. J Am Acad Child Adolesc Psychiatry. 2004;43(10):1243–1249. doi: 10.1097/01.chi.0000135621.23145.05. [DOI] [PubMed] [Google Scholar]
- 21.Huang J.S., Dillon L., Terrones L., Schubert L., Roberts W., Finklestein J. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatr Blood Canc. 2014;61(5):894–900. doi: 10.1002/pbc.24937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunin-Batson A., Steele J., Mertens A., Neglia J.P. A randomized controlled pilot trial of a Web-based resource to improve cancer knowledge in adolescent and young adult survivors of childhood cancer. Psycho Oncol. 2016;25(11):1308–1316. doi: 10.1002/pon.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sander Wint S., Eshelman D., Steele J., Guzzetta C.E. Effects of distraction using virtual reality glasses during lumbar punctures in adolescents with cancer. Oncol Nurs Forum. 2002;29(1):E8–E15. doi: 10.1188/02.onf.e8-e15. [DOI] [PubMed] [Google Scholar]
- 24.Alemi M., Ghanbarzadeh A., Meghdari A., Moghadam L.J. Clinical application of a humanoid robot in pediatric cancer interventions. International Journal of Social Robotics. 2016;8(5):743–759. [Google Scholar]
- 25.Li W.H., Chung J.O., Ho E.K. The effectiveness of therapeutic play, using virtual reality computer games, in promoting the psychological well-being of children hospitalised with cancer. J Clin Nurs. 2011;20(15–16):2135–2143. doi: 10.1111/j.1365-2702.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 26.Campo R.A., Bluth K., Santacroce S.J., Knapik S., Tan J., Gold S. A mindful self-compassion videoconference intervention for nationally recruited posttreatment young adult cancer survivors: feasibility, acceptability, and psychosocial outcomes. Support Care Canc. 2017;25(6):1759–1768. doi: 10.1007/s00520-017-3586-y. [DOI] [PubMed] [Google Scholar]
- 27.Hooke M.C., Gilchrist L., Tanner L., Hart N., Withycombe J.S. Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatr Blood Canc. 2016;63(4):684–689. doi: 10.1002/pbc.25860. [DOI] [PubMed] [Google Scholar]
- 28.Jibb L.A., Stevens B.J., Nathan P.C., Seto E., Cafazzo J.A., Johnston D.L. Implementation and preliminary effectiveness of a real-time pain management smartphone app for adolescents with cancer: a multicenter pilot clinical study. Pediatr Blood Canc. 2017;64(10) doi: 10.1002/pbc.26554. [DOI] [PubMed] [Google Scholar]
- 29.Schneider S.M., Workman M.L. Effects of virtual reality on symptom distress in children receiving chemotherapy. Cyberpsychol Behav. 1999;2(2):125–134. doi: 10.1089/cpb.1999.2.125. [DOI] [PubMed] [Google Scholar]
- 30.Seitz D.C., Knaevelsrud C., Duran G., Waadt S., Loos S., Goldbeck L. Efficacy of an internet-based cognitive-behavioral intervention for long-term survivors of pediatric cancer: a pilot study. Support Care Canc. 2014;22(8):2075–2083. doi: 10.1007/s00520-014-2193-4. [DOI] [PubMed] [Google Scholar]
- 31.Robison L.L., Hudson M.M. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Canc. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohr D.C., Burns M.N., Schueller S.M., Clarke G., Klinkman M. Behavioral intervention technologies: evidence review and recommendations for future research in mental health. Gen Hosp Psychiatr. 2013;35(4):332–338. doi: 10.1016/j.genhosppsych.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hockenberry M.J., Hooke M.C., Gregurich M., McCarthy K., Sambuco G., Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010;37(1):E16–E27. doi: 10.1188/10.Onf.E16-e27. [DOI] [PubMed] [Google Scholar]
- 34.Hockenberry M.J., Hooke M.C., Rodgers C., Taylor O., Koerner K.M., Mitby P. Symptom trajectories in children receiving treatment for leukemia: a latent class growth analysis with multitrajectory modeling. J Pain Symptom Manag. 2017;54(1):1–8. doi: 10.1016/j.jpainsymman.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laird B.J., Scott A.C., Colvin L.A., McKeon A.L., Murray G.D., Fearon K.C. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manag. 2011;42(1):1–11. doi: 10.1016/j.jpainsymman.2010.10.261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.