Abstract
Recent studies have highlighted an important role for lysophosphatidylcholine acyltransferase 3 (LPCAT3) in controlling the PUFA composition of cell membranes in the liver and intestine. In these organs, LPCAT3 critically supports cell-membrane-associated processes such as lipid absorption or lipoprotein secretion. However, the role of LPCAT3 in macrophages remains controversial. Here, we investigated LPCAT3's role in macrophages both in vitro and in vivo in mice with atherosclerosis and obesity. To accomplish this, we used the LysMCre strategy to develop a mouse model with conditional Lpcat3 deficiency in myeloid cells (Lpcat3KOMac). We observed that partial Lpcat3 deficiency (approximately 75% reduction) in macrophages alters the PUFA composition of all phospholipid (PL) subclasses, including phosphatidylinositols and phosphatidylserines. A reduced incorporation of C20 PUFAs (mainly arachidonic acid [AA]) into PLs was associated with a redistribution of these FAs toward other cellular lipids such as cholesteryl esters. Lpcat3 deficiency had no obvious impact on macrophage inflammatory response or endoplasmic reticulum (ER) stress; however, Lpcat3KOMac macrophages exhibited a reduction in cholesterol efflux in vitro. In vivo, myeloid Lpcat3 deficiency did not affect atherosclerosis development in LDL receptor deficient mouse (Ldlr−/−) mice. Lpcat3KOMac mice on a high-fat diet displayed a mild increase in hepatic steatosis associated with alterations in several liver metabolic pathways and in liver eicosanoid composition. We conclude that alterations in AA metabolism along with myeloid Lpcat3 deficiency may secondarily affect AA homeostasis in the whole liver, leading to metabolic disorders and triglyceride accumulation.
Supplementary key words: phospholipid, arachidonic acid, macrophages, atherosclerosis, steatosis, lysophosphatidylcholine acyltransferase 3 (LPCAT3), lipid metabolism, obesity, inflammation, insulin resistance
Abbreviations: AA, arachidonic acid; LPCAT3, lysophosphatidylcholine acyltransferase 3; LPLAT, lyso-PL-acyltransferase; LPS, lipopolysaccharide; LXR, liver X receptor; PC, phosphatidylcholine; PL, phosphatidylinositol; pPE, plasmalogen; PS, phosphatidylserine
Phospholipids (PLs) are continuously remodeled in a succession of deacylation and reacylation reactions called the Lands cycle (1). Turnover of fatty acids (FAs) at sn-2 position of PLs is mediated by the opposite actions of phospholipases A2 and lyso-PL-acyltransferases (LPLATs). Each LPLAT action is tissue- and substrate-dependent (2, 3). One of these enzymes, the lysophosphatidylcholine acyltransferase 3 (LPCAT3), is highly expressed in the liver and the intestine but also in macrophages (4, 5, 6). It has been shown that LPCAT3 promotes the insertion of polyunsaturated fatty acid (PUFAs), mainly arachidonic acid (AA), at the sn-2 position of cellular PLs (5, 6). LPCAT3 expression is dynamically regulated by nuclear receptors such as liver X receptors (LXRs) and peroxisome proliferator-activated receptors (PPARs) (4, 7, 8). Deletion of LPCAT3 decreases the abundance of PUFAs within PLs leading to alterations of the physicochemical properties of cell membranes. In mouse models, Lpcat3 deletion is associated with alterations of several membrane-associated processes such as lipoprotein assembly and secretion by hepatocytes (5, 6), dietary lipid absorption (5, 6, 9), and SREBP1c cleavage (10). An acute inhibition of Lpcat3 was shown to promote ER stress and inflammation in the liver (8), whereas it was not observed in Lpcat3−/− mice. In the mouse, constitutive Lpcat3 deficiency is lethal few days after birth probably due to the malabsorption of dietary lipids (5, 9, 11). While these studies focused mainly on the liver and intestine, the impact of LPCAT3 on macrophage functions is more controversial. While acute Lpcat3 inhibition in murine peritoneal macrophages was shown to potentiate the inflammatory response to lipopolysaccharides (LPSs) (4), siRNA-mediated knockdown of LPCAT3 in human primary macrophages did not affect the overall inflammatory response but decreased eicosanoid secretion, probably due to a depletion of AA in membrane phospholipids (8). Phenotypic analysis of Lpcat3-deficient mouse macrophages also revealed some discrepancies between the studies. Indeed, a complete Lpcat3 deficiency in fetal liver-derived macrophages did not affect the inflammatory response or ER stress but was shown to affect eicosanoid secretion and cholesterol homeostasis (12). The latter point was associated with an inhibition of cholesterol efflux pathways. In contrast, in bone-marrow-derived macrophages (BMDMs) from Lpcat3-Flox/LysM-Cre mice presenting with a 20% residual Lpcat3 activity, it was observed an increase in Il1b mRNA levels and IL-6 and TNF-α secretion following LPS stimulation while some markers of ER stress were significantly decreased (13). Finally, in a study using the same Lpcat3-Flox/LysM-Cre mouse model, Lpcat3 deficiency did not affect Il1b mRNA levels and significantly reduced Cox2 mRNA levels following LPS stimulation (14). To clarify the function of LPCAT3 in macrophages in vitro and in vivo, we generated a mouse model with a conditional Lpcat3 deficiency in myeloid cells (Lpcat3KOMac mice) presenting with an 80% reduction of Lpcat3 expression in macrophages. Moreover, we investigated the impact of macrophage Lpcat3 deficiency in mouse models of atherosclerosis and obesity/hepatic steatosis. Here, we showed that a partial deletion of Lpcat3 in macrophages significantly affects the FA composition of all phospholipid subclasses as well as the distribution of AA within the cellular lipids, but did not alter the inflammatory response in vivo or in vitro. While Lpcat3 macrophage deficiency did not affect atherosclerosis development, Lpcat3KOMac mice presented with a mild increase in hepatic steatosis under a high-fat diet (HFD) that was associated with alterations of several metabolic pathways and liver eicosanoid composition.
Materials and methods
Generation of myeloid-cell-specific Lpcat3-deficient mice
Lpcat3fl/fl mice (12) were crossed with LysMCre/+ transgenic mice to obtain Lpcat3fl/fl/LysMCre/+ (Lpcat3KOMac) and Lpcat3fl/fl/LysM+/+ littermate (WT). We used male and female mice on a C57BL/6N background. All animal procedures were performed in accordance with institutional guidelines and approved by the University of Burgundy's Ethics Committee on the Use of Laboratory Animals (protocol number 8381). At the age of 8–12 weeks old, mice were either maintained on a chow diet (CD) (A3, Safe) or fed an HFD for 16 weeks (60% fat diet, D12492, Ssniff) or a western diet for 12 weeks (TD88137, Harlan Teklad).
Bone marrow transplantation
Eight-week-old Ldlr−/− mice were lethally irradiated with 1,000 rads (10 Gy) before transplantation. Recipient mice were injected with about 2 × 106 bone-marrow-derived monocytes through the tail vein. Recipient Ldlr−/− mice were given acidified water (pH 4.5) containing enrofloxacin 0.25% for 2 weeks after transplantation.
Atherosclerosis study
All experiments were performed according to already published protocols (12).
In vivo metabolic tests
Glucose (2 g/kg, oral administration), pyruvate (2 g/kg, i.p.), and insulin (0.4–0.8 U/kg, i.p.) tolerance tests were performed after a 6-h fast (OGTT, ITT) or overnight fast (PTT). Glycemia is measured with glucometer (Accu-Chek Performa).
LPS treatment
LPSs from Escherichia coli 055:B5 (L2880, Sigma) were solubilized in saline 0.9% and injected i.p. in mice at 1 mg/kg. Treatment of culture cells was performed at a concentration of 100 ng/ml.
BMDM preparation
Anesthetized mice were euthanized by cervical dislocation. Bone marrow in femur and tibia was flushed, and 300,000 bone marrow cells were implanted in 12-well plates. Cells were treated during 5–7 days with human M-CSF (130-096-492, Miltenyi) until full macrophage differentiation.
Isolation of Kupffer cells and primary hepatocytes
Mice were anesthetized, and a two-step collagenase perfusion of the liver was performed as previously described (15). Digested livers were centrifugated (30 g, 3 min, 4°C). Briefly, cell pellets containing primary hepatocytes were washed and divided for mRNA analysis and metabolic activity assay (Substrate palmitate-BSA FAO Seahorse XF, Agilent) on SeaHorse XFe96 Analyzer (Agilent). Primary hepatocytes were plated in FAO Assay Buffer according to manufacturer protocol (Krebs Henseleit Buffer: 111 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 2 mM MgSO4, 1.2 mM NaH2PO4, 2.5 mM glucose, 0.5 mM carnitine, and 5 mM HEPES) and were treated with control BSA or 0.17 mM palmitate:BSA (6:1 ratio) prior to the XF assay being initiated (t = 0). Oxygen consumption rate was measured in basal conditions and after injections of oligomycin, FCCP, and Rotenone/Antimycin A. Supernatants containing Kupffer cells were used for cell sorting using MACS (Anti-F4/80 MicroBeads UltraPure, mouse, Miltenyi) and analyzed by RNA sequencing (Genewiz, Germany, GEO number: GSE146004).
Lipidomic analyses
Phosphatidylcholine, phosphatidylethanolamine, cholesterol, and FA analyses were performed at the lipidomic platform of Dijon (France) according to protocols already published (12). Phosphatidylinositols and phosphatidylserines were analyzed by LCMS/MS using the same chromatographic conditions as previously described (12). Acquisition was performed on an Agilent 6460 QqQ mass spectrometer in negative selected reaction monitoring ion mode (source temperature 325°C, nebulizer gas flow rate 10 L/min, sheath gas flow 11 L/min, temperature 300°C, capillary 3500 V, nozzle 1000 V). Fragmentor was set up at 172 V and 150 V for phosphatidylinositols and phosphatidylserines, respectively. Collision energy was set up at 50 V and 19 V for phosphatidylinositols and phosphatidylserines, respectively. Each glycerophospholipid was semiquantitated by calculating their response ratio with regard to their respective internal standard.
Biochemical analyses
Plasma insulin and GLP-1 were measured with ELISA kit (STELLUX® Chemiluminescent Rodent Insulin ELISA ALPCO and EZGLP1T-36K, Millipore, respectively). Triglycerides (TGs), cholesterol, and free fatty acid (FFA) concentrations were measured by enzymatic methods (Triglycerides FS, Cholesterol FS, NEFA FS, Diasys). Cytokines were measured with a Milliplex MAP 5-Plex Kit using mouse cytokine/chemokine magnetic bead panel (MCYTOMAG-70K, Millipore), according to the manufacturer's protocol, and using a LuminexR apparatus (Bio-Plex 200, Bio-Rad).
Cholesterol efflux
Cholesterol efflux experiments were performed according to published protocol (12).
Real-time quantitative PCR
Tissues were immediately frozen in liquid nitrogen and stored at –80°C. Cell culture lysates were directly stored at –80°C. Total RNA was isolated using RNeasy Mini Kit (74106, Qiagen) and quantified by spectrophotometer (Nanodrop 1000, Thermo Scientific). In total, 100–1000 ng RNA was reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Multiscribe® reverse transcriptase, 4368813, Applied Biosystems), and quantitative PCR were performed using StepOnePlus (Real-Time PCR System, Applied Biosystems) and SYBRGreen® (4367659, Applied Biosystems) technologies. The mRNA levels were normalized with housekeeping gene Rplp0 and expressed as relative expression using the 2−ΔΔCt method.
Statistical analyses
Data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism. To decide whether to use parametric or nonparametric statistics, the normality of distributions was assessed with the Shapiro-Wilk test (under n = 7, distributions were considered to be nonnormal). Statistical significance of differences between two groups was evaluated with the Mann-Whitney U test or the Student's t-test. A value of P < 0.05 was considered statistically significant (NS, not significant; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001).
Results
Lipidomic characterization of Lpcat3KOMac cells
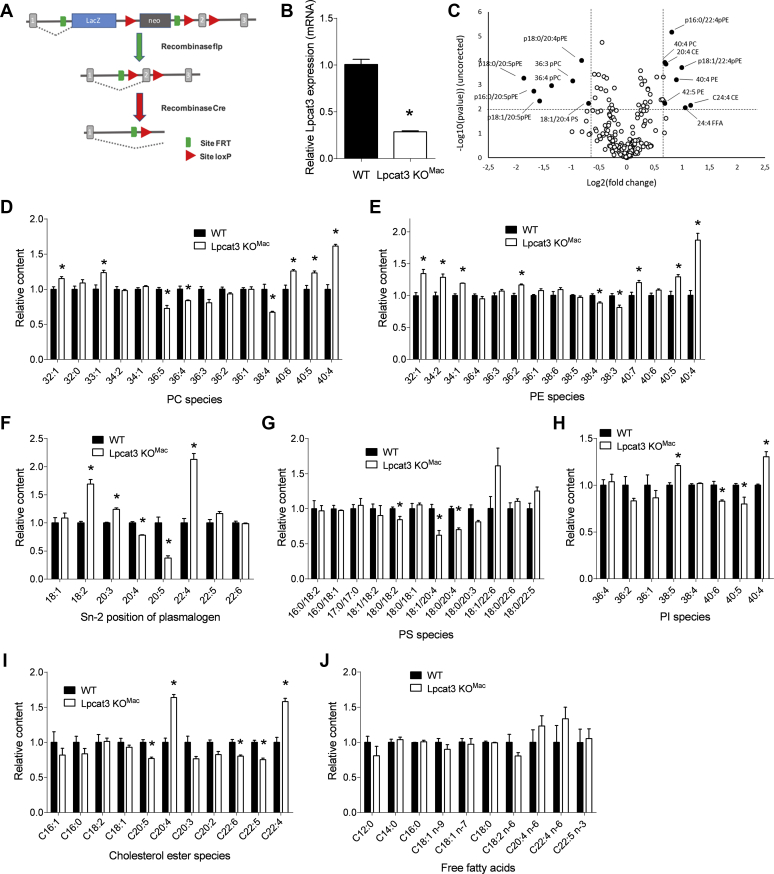
We generated a transgenic model of mice deficient for Lpcat3 in myeloid cells (Lpcat3KOMac mice) by crossing Lpcat3-Flox mice with LysMCre transgenic mice (Fig. 1A). In isolated BMDMs, Lpcat3 mRNA levels were reduced by approximately 75% in Lpcat3KOMac cells as compared with controls (Lpcat3-Flox) (Fig. 1B). To analyze the impact of Lpcat3 deficiency on the lipid composition of BMDMs, we conducted a targeted lipidomic analysis of 244 molecules including major phospholipid subclasses (phosphatidylcholines [PCs], phosphatidylserines [PSs], phosphatidylethanolamines [PEs], phosphatidylinositols [PIs], plasmalogens [pPEs], total FAs, FFAs, and cholesteryl esters) (supplemental Fig. S1). By using a P value of 0.01 and ±0.6 log-fold changes as a cutoff, we found that the levels of 14 molecules were significantly altered in Lpcat3KOMac cells (Fig. 1C). As expected, C20:4 n-6 and C20:5 n-3 containing phospholipids were among the molecules that were significantly reduced in Lpcat3KOMac macrophages, while C22:4 containing phospholipids and C20:4 cholesteryl esters were increased. Analysis of phospholipid subclasses revealed that in addition to PCs, Pes, and pPEs (Fig. 1D–F) that were previously characterized as LPCAT3 substrates, the composition of PSs (Fig. 1G) and PIs (Fig. 1H) was also significantly affected by Lpcat3 deficiency. C20:4 depletion in phospholipids was associated with a redistribution of AA toward cholesteryl esters (Fig. 1I), as previously observed (12). However, as compared with total Lpcat3 deficiency (12), changes in FA composition of PLs were less pronounced in Lpcat3KOMac cells, and there was no significant increase in non-esterified FA levels (Fig. 1J) including AA and C22:4 n-6 (i.e., a direct elongation product of AA) as it was the case in Lpcat3−/− macrophages (12). Nevertheless, our data demonstrate that even a partial Lpcat3 deficiency markedly affects AA homeostasis and distribution in macrophages.
Fig. 1.
Generation of Lpcat3KOMac mice and lipidomic characterization of Lpcat3-deficient macrophages. A: Lpcat3 targeting vector. A gene-trap LacZ cassette is located downstream of exon 2 of the Lpcat3 gene. B: Relative Lpcat3 mRNA levels in macrophages derived from WT and Lpcat3KOMac mice (four independent mice in each group). Data are expressed as mean ± SEM (∗P < 0.05 vs. WT Mann-Whitney test). C: Changes of lipidomic profile of Lpcat3KOMac versus WT macrophages (four independent mice in each group, P value at 0.01 and ±0.6 log-fold changes as cut off). D–J: Fatty acid composition of phosphatidylcholines (D), phosphatidylethanolamines (E), plasmalogens (F), phosphatidylserines (G), phosphatidylinositols (H), cholesterol esters (I), and free fatty acids (J) of WT and Lpcat3KOMac macrophages (n = 4 in each group). Data are expressed as mean + SEM (∗P < 0.05 vs. WT Mann-Whitney test).
Inflammatory response and ER stress of Lpcat3KOMac cells
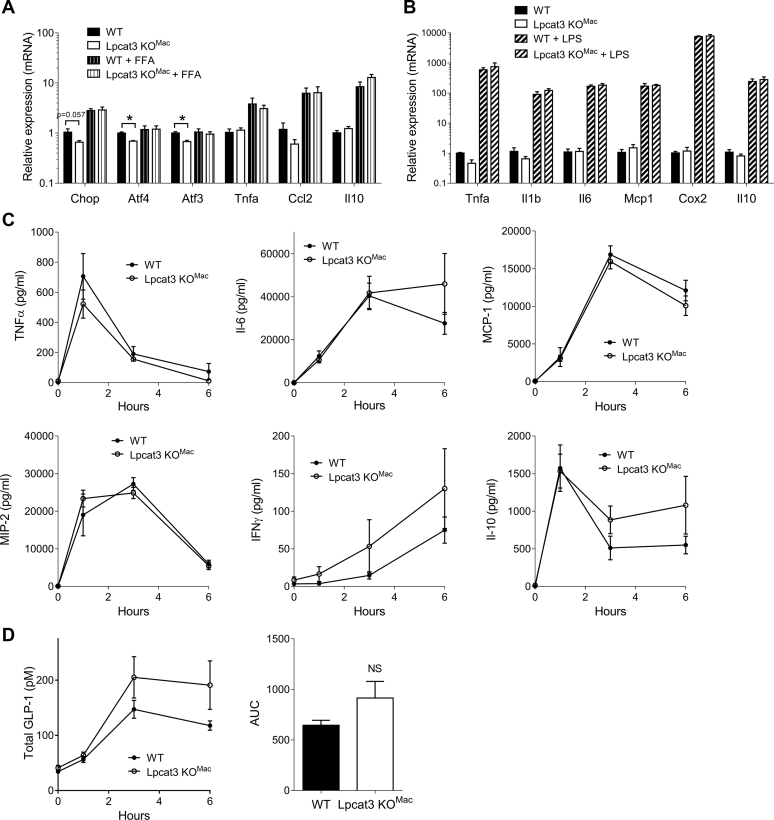
It has been suggested that LPCAT3-dependent membrane remodeling may affect ER stress (8) and inflammation (13). We evaluated the impact of Lpcat3 deficiency on these two biological processes under both basal and stimulated conditions. Interestingly, Lpcat3KOMac macrophages presented a slight but significant decrease in the expression of ER stress markers at basal state; however, after loading of the macrophages with saturated FFAs to induce ER stress, there were no differences between the two genotypes (Fig. 2A). No differences were observed between WT and Lpcat3KOMac cells regarding inflammatory gene expression under either control, or FFA loading, or LPS-stimulated conditions (Fig. 2A, B). To evaluate the inflammatory response in vivo, WT and Lpcat3KOMac mice were challenged with LPS injection and plasma cytokines were evaluated up to 6 h after LPS injection. In accordance with our in vitro observations, we did not find any differences in cytokine secretion (Fig. 2C). Furthermore, secretion of GLP-1, which is produced by enteroendocrine cells in response to LPS, was not significantly increased in Lpcat3KOMac mice treated with LPS (Fig. 2D). Our results suggest therefore that while ER stress could be slightly reduced in Lpcat3KOMac macrophages, there is no obvious impact on acute inflammatory response in vitro and in vivo.
Fig. 2.
ER stress and inflammatory response in Lpcat3KOMac mice. A: Relative mRNA levels of ER stress markers or inflammatory genes in macrophages from WT and Lpcat3KOMac mice and treated or not with 200 μM free fatty acids (oleate and palmitate) (n = 4 independent mice in each group). B: Relative expression of pro- or anti-inflammatory genes in macrophages from WT and Lpcat3KOMac mice treated or not with 100 ng/ml LPS (n = 4 independent mice in each group) Data are expressed as mean + SEM. (∗P < 0.05 vs. WT Mann-Whitney test by treatment condition). C, D: Plasma concentration of cytokines (C) and total GLP-1 (D) in WT and Lpcat3KOMac mice treated with 1 mg/kg LPS (n = 6 and 5, respectively). Data are expressed mean ± SEM (∗P < 0.05 vs. WT Mann-Whitney test for AUC).
Partial Lpcat3 deficiency restricted to myeloid cells does not impact atherosclerosis development
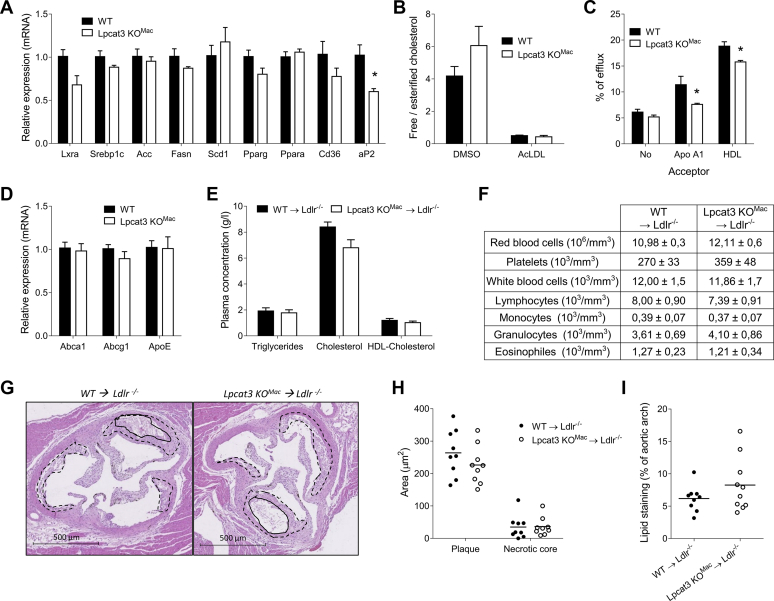
Next, we wanted to address the impact of Lpcat3 deficiency on macrophage lipid homeostasis and atherosclerosis development. Targeted transcriptomic analysis of Lpcat3KOMac BMDMs did not revealed major alterations in genes involved in FA and cholesterol metabolism at the exception of Ap2 (Fabp4) that was significantly decreased in Lpcat3KOMac macrophages (Fig. 3A). Interestingly, there was a significant decrease in cholesterol efflux toward ApoA1 and HDL in Lpcat3KOMac macrophages as compared with WT cells (Fig. 3C). Since Abca1 and Abcg1 mRNA levels were similar in WT and Lpcat3KOMac macrophages (Fig. 3D), these data suggest that Lpcat3 deficiency may affect cholesterol efflux by altering cell membrane lipid composition. To evaluate the impact of Lpcat3 macrophage deficiency on atherosclerosis development, Ldlr−/− mice were lethally irradiated and then transplanted with hematopoietic cells harvested from WT or Lpcat3KOMac bone marrows. After 4 weeks of recovery, the mice were fed with a Western-type diet (WTD) for 14 weeks. There was no difference in weight gain during the diet (data not shown), and the two groups of mice displayed similar plasma lipid concentrations (Fig. 3E). There was no difference in peripheral blood leukocyte counts, including monocytes, after 14 weeks of WTD (Fig. 3F). Analysis of atherosclerotic lesions after 14 weeks revealed similar lesion sizes in aortic roots in mice reconstituted with Lpcat3KOMac or WT bone marrow cells. There was also no difference in the necrotic core area (Fig. 3G, H). No difference was observed regarding atherosclerotic lesions in aortic arches (Fig. 3I).
Fig. 3.
Partial Lpcat3 deficiency restricted to myeloid cells does not induce atherosclerosis. A: Relative mRNA levels of genes involved in lipid metabolism (n = 4 vs. 4). B: Ratio of free to esterified cholesterol in WT and Lpcat3KOMac mouse-derived macrophages treated or not with acetylated LDL (n = 4 vs. 4 independent mice in each group). C: Cholesterol efflux with lipid-free ApoA-I or HDL was assessed in [3H] cholesterol-acetylated LDL loaded macrophages, n = 3 independent experiments. D: Relative mRNA levels of Abca1, Abcg1 and ApoE (n = 9 in each group) in WT and Lpcat3KOMac mouse-derived macrophages. E: Plasma lipid parameters from recipient Ldlr−/− mice transplanted with WT and Lpcat3KOMac BMDM cells at 14 weeks of Western-type diet (n = 9 in each group). F: Blood cell counts from recipient Ldlr−/− mice transplanted with WT and Lpcat3KOMac bone marrow cells (n = 9 in each group). G, H: HE staining of aortic valves from Ldlr−/− recipient mice fed with a Western-type diet for 14 weeks. G: Dotted line, atheroma plaque; continuous line, necrotic core area. H: Analysis of plaque and necrotic core size. I: % of Oil Red O stained area in aortic arches of Ldlr−/− recipient mice fed a 12 week WTD. Values are expressed mean + SEM (∗P < 0.05, ∗∗P < 0.01 vs. WT Mann-Whitney test).
Weight gain is not affected by a myeloid Lpcat3 deficiency under standard diet
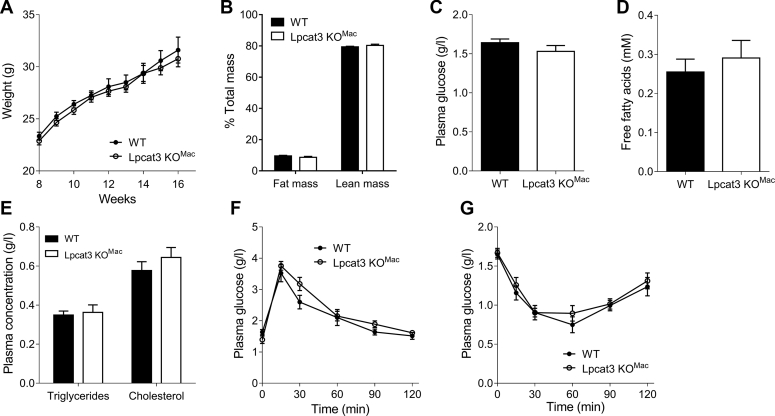
Obesity is another chronic inflammatory disease in which macrophages are responsible for progression of chronic inflammation and insulin resistance (16). We investigated the impact of macrophage-specific Lpcat3 deficiency on progression of obesity. First, under a CD, we observed that Lpcat3KOMac and WT mice grow at a similar rate (Fig. 4A). No changes in fat or lean mass were observed (Fig. 4B). No differences were observed in blood fasting glucose (Fig. 4C), FFAs (Fig. 4D) or TGs, and cholesterol (Fig. 4E) between Lpcat3KOMac and WT mice. Glucose metabolism was assessed through in vivo oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). As shown in Figure 4F, no differences were observed with these two different tests. According to these results, Lpcat3KOMac mice fed a CD thrive normally without obvious alterations in glucose or lipid metabolism.
Fig. 4.
Lpcat3KOMac mice grow normally under a chow diet. A: Weight gain of mature WT and Lpcat3KOMac mice fed a chow diet (n = 7 vs. 13, respectively). B: Fat mass and lean mass of WT and Lpcat3KOMac mice after 8 weeks of chow diet (n = 7 vs. 13, respectively). C–E: Plasma glucose (C), free fatty acids (D) and triglycerides, and cholesterol (E) were assessed in 6 h fasting WT and Lpcat3KOMac mice (n = 6 in each group). F, G: Oral glucose tolerance test (F) and insulin tolerance test (G) of 6 h fasting WT and Lpcat3KOMac mice after 8 weeks of chow diet (n = 6 in each group). Data are expressed mean ± SEM (∗P < 0.05 vs. WT Mann-Whitney test or Mann-Whitney test for AUC).
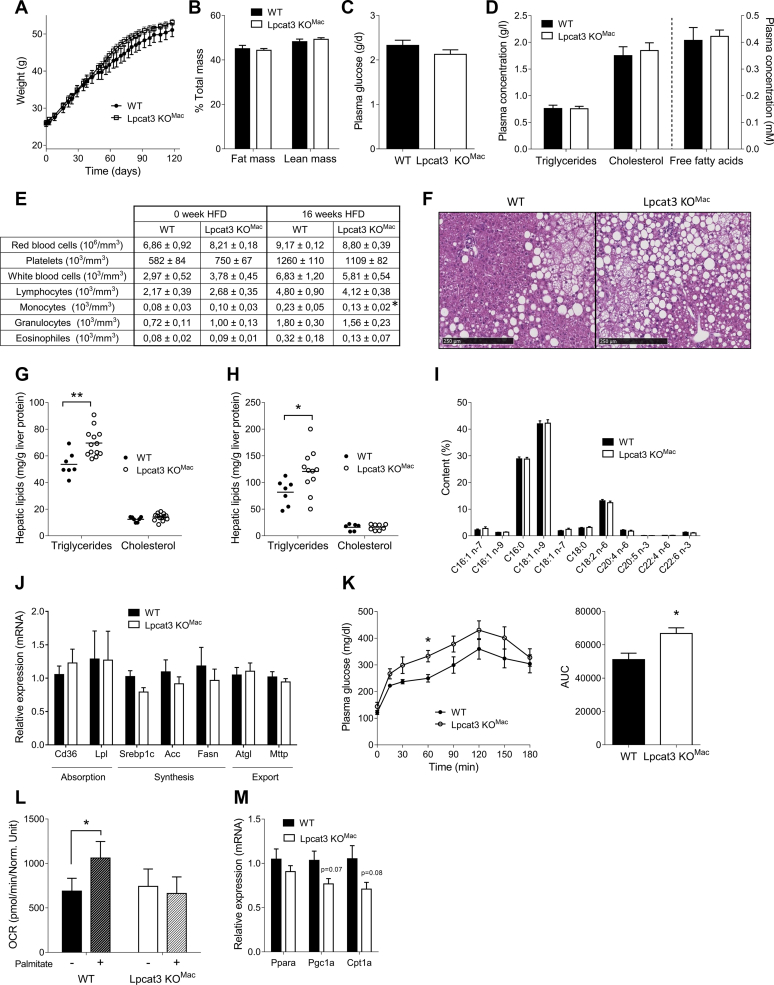
Lpcat3KOMac mice develop hepatic steatosis under high-fat diet
The impact of an HFD on obesity progression was then explored in Lpcat3KOMac mice. While the % of fat mass increased dramatically after 4 months of HFD, body mass and body composition (Fig. 5A, B) as well as food intake (supplemental Fig. S2A) were not different between the two genotypes (Fig. 5B). Lpcat3 deficiency did not affect the blood parameters measured. Indeed, fasting plasma glucose (Fig. 5C), TGs, cholesterol, and FFA concentrations (Fig. 5D) were unchanged compared with WT mice. Interestingly, number of circulating monocytes was reduced in Lpcat3KOMac mice at the end of the diet, whereas it was not different at the beginning (Fig. 5E). As observed under a CD, no differences were observed with OGTT or ITT (supplemental Fig. S2B–D). Fat storage was then assessed in the adipose tissue and liver. While no difference was observed in the adipose tissue mass, adipocyte size, or expression of genes involved in adipocyte metabolism (supplemental Fig. S3), livers of Lpcat3KOMac mice displayed significant alterations. Indeed, histological sections of livers from Lpcat3KOMac mice showed higher macrovesicular steatosis as compared with WT mice (Fig. 5F). Liver lipid content was measured, and we observed a mild but significant increase of TGs in both males and females Lpcat3KOMac mice; in contrast, cholesterol content remained unchanged (Fig. 5G, H). The liver FA profile was similar in the two groups of mice, with palmitic acid (C16:0) and oleic acid (C18:1) representing the majority of the FAs (Fig. 5I). No changes in the expression of genes involved in lipid absorption, synthesis, or export were observed in the liver (Fig. 5J), whereas a pyruvate tolerance test revealed an increase in the conversion of pyruvate to glucose in Lpcat3KOMac mice compared with WT mice (Fig. 5K). FA oxidation was investigated in primary hepatocytes isolated after 16 week HFD (Fig. 5L). An increase of oxygen consumption rate (OCR) was observed in hepatocytes from WT mice following palmitate addition, whereas no changes were observed in hepatocytes from Lpcat3KOMac mice. Genes involved in the FA oxidation pathway were not significantly reduced in Lpcat3KOMac hepatocytes despite a strong trend (Fig. 5M). In conclusion, under an HFD, Lpcat3KOMac mice suffer from hepatic steatosis that seemed associated with decreased FA oxidation and increased gluconeogenesis.
Fig. 5.
Lpcat3KOMac mice suffer from hepatic steatosis when fed a high-fat diet. A: Weight gain of mature WT and Lpcat3KOMac mice fed a high-fat diet (n = 7 and 13, respectively). B: Fat mass and lean mass of WT and Lpcat3KOMac mice after 16 weeks of high-fat diet (n = 7 and 13, respectively). Plasma glucose (C), free fatty acids and triglycerides, and cholesterol (D) were assessed in 6 h fasting WT and Lpcat3KOMac mice at the end of the diet (n = 7 and 13, respectively). E: Blood cell counts at the beginning and the end of HFD (n = 7 and 13, respectively). F: HE staining of liver of WT and Lpcat3KOMac mice after 16 weeks of high-fat diet. G, H: Measurements of lipid content in the liver of males (G, n = 7 vs. 13) and females (H, n = 7 vs. 11). I: Total fatty acid content in the liver of WT and Lpcat3KOMac mice (n = 7 vs. 13). J: Relative mRNA levels of genes involved in liver lipid metabolism pathways (n = 7 vs. 13). K: Pyruvate tolerance test of overnight fasting WT and Lpcat3KOMac mice and area under cover of this PTT. L: Oxygen consumption rate of primary hepatocytes of WT and Lpcat3KOMac mice, treated or not with palmitate. M: Liver relative mRNA levels of genes involved in fatty acid oxidation at the end of the HFD (n = 7 vs. 13). Data are expressed mean ± SEM (∗P < 0.05 vs. WT Mann-Whitney test).
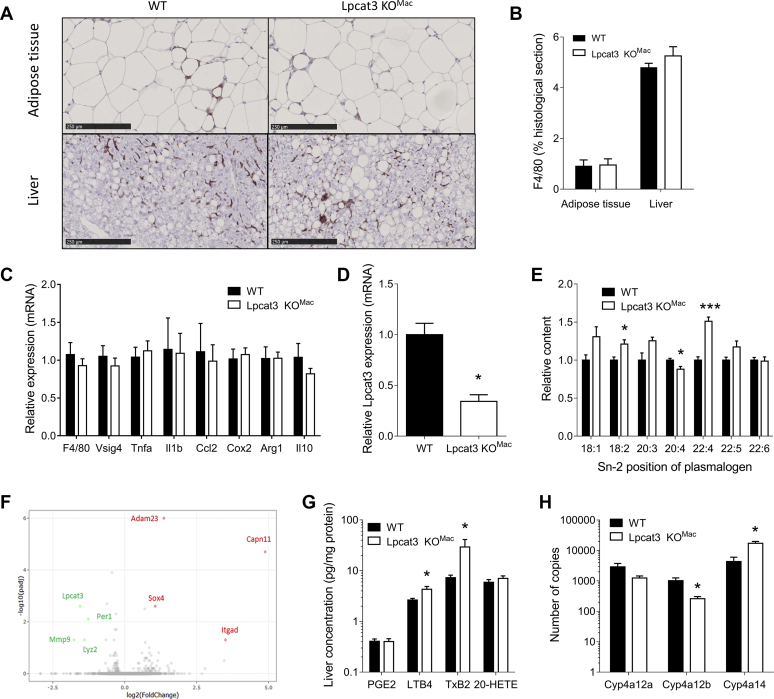
Lipidomic and transcriptomic analyses reveal significant alterations of AA metabolism in the liver from Lpcat3KOMac mice
Histological sections stained with F4/80 antibodies were performed on the adipose tissue and liver after 4 months of HFD (Fig. 6A). There was no difference in macrophage infiltration in the adipose tissue and in the liver of Lpcat3KOMac mice as compared with WT mice (Fig. 6B). Furthermore, analysis of the sections did not reveal signs of liver inflammation and expressions of inflammatory genes were similar in the liver of Lpcat3KOMac mice fed an HFD as compared with control mice (Fig. 6C). Liver F4/80-positive cells were isolated by magnetic cell sorting (MACS). As expected, myeloid cells from Lpcat3KOMac mice displayed reduced Lpcat3 mRNA levels (Fig. 6D). Lipidomic analysis confirmed the phenotype with a decrease of C20:4 n-6 and an increase of C18:2 n-6 and C22:4 n-6 at the sn-2 position of plasmalogens (Fig. 6E). The transcriptome of liver F4/80 positive cells was investigated by a global RNAseq approach. Surprisingly, a very low number of genes were found to be differentially expressed between the two genotypes, among them Lpcat3 and Lyz2 as expected. Notably, there was no difference in inflammatory genes as assessed by a PCR approach (Fig. 6F). Since F4/80 positive cells displayed an alteration in their AA composition, we measured the concentration of AA-derived mediators in the whole liver. Interestingly, eicosanoid profile was altered in the liver of Lpcat3KOMac mice with significant increases in leukotriene B4 (LTB4) and thromboxane B2 (TxB2) concentrations (Fig. 6G). While there were no changes in the expression of enzymes involved in leukotriene or thromboxane pathways in the Kupffer cells from Lpcat3KOMac mice, analysis of the whole liver tissue revealed significant alterations of CYP450 enzymes involved in AA metabolism (17) with a significant decrease of Cyp4a12b and increase of Cyp4a14 mRNA levels (Fig. 6H). While these enzymes are not directly involved in eicosanoid synthesis, these data come in further support of an altered AA homeostasis in the liver of Lpcat3KOMac mice.
Fig. 6.
Mechanisms involved in hepatic steatosis progression in Lpcat3KOMac mice when fed a high-fat diet for 16 weeks. A, B: F4/80 staining in histological sections of adipose tissue and liver of Ctrl and Lpcat3 KOMac (n = 7 vs. 13, respectively). C: Liver mRNA levels of inflammatory genes of wild-type and Lpcat3KOMac mice (n = 7 vs. 13, respectively). D: Relative Lpcat3 mRNA levels of in isolated Kupffer cells (n = 3 in each group). E: Relative content of fatty acid at the sn-2 position of pPE from isolated Kupffer cells after HFD. Data are expressed as a % of total pPE and are normalized as 1 in the WT group (n = 3 in each group). F: Volcano plot of differentially expressed genes in Kupffer cells at the end of the diet (n = 3 in each group). G: Eicosanoid content in the liver of WT and Lpcat3KOMac mice (n = 7 vs. 13, respectively). H: Relative mRNA levels of genes involved in Cytochrome P450 pathway in the whole liver (n = 4 in each group). Data are expressed as mean + SEM (∗P < 0.05 vs. WT Mann-Whitney test).
Discussion
Recent studies have highlighted the major role of LPCAT3 in controlling the PUFA composition of cell membranes in the liver and intestine with dramatic consequences on cell-membrane-associated processes such as lipid absorption, lipoprotein secretion, and SREBP cleavage (5, 6, 11). In contrast, the role of LPCAT3 in myeloid cells and macrophages remains unclear (4, 8, 12, 13). In the present study, we developed a mouse model with conditional Lpcat3 deficiency in myeloid cells by using the LysMCre strategy. Our aim was to investigate the function of LPCAT3 in macrophages in vitro but also in vivo in experimental models of atherosclerosis and obesity. Indeed, chronic low-grade inflammation mediated in part by macrophages and myeloid cells is known to play a key role in these two cardiometabolic diseases (18, 19).
As it is often the case with similar conditional models (20), crossing the Lpcat3flox/flox mice with the LysMcre mice led to an incomplete inactivation of the Lpcat3 gene in myeloid cells with a residual approximately 25% expression that was observed either in vitro or in vivo. Thus, our Lpcat3KOMac model should be considered as model of partial Lpcat3 deficiency in myeloid cells with significant residual Lpcat3 activity. As a consequence, while the changes of phospholipid composition were broadly similar to those in fetal liver cell-derived macrophages with total Lpcat3 deficiency (12) they were much less pronounced. Nevertheless, our data demonstrate that even a partial Lpcat3 deficiency has a marked impact on the lipidomic profile of macrophages, thus underlying the major role of LPCAT3 in PL remodeling and PUFA metabolism in macrophages. While we confirm the reduction of EPA and AA in PCs, Pes, and pPEs as observed in Lpcat3−/− macrophages, we extend these observations to PIs and PSs. However, LPCAT3 may not use LysoPI and LysoPS as direct substrates. Rather, our data suggest that the alterations in the FA composition of PCs and PEs induced by Lpcat3 deficiency secondarily affect other phospholipid subclasses. As previously observed, the reduced incorporation of arachidonate in PLs was associated with its redistribution toward other cellular lipids, such as cholesteryl esters. Increased C22:4 levels in different lipid subclasses (PEs, cholesteryl esters) were also observed suggesting a compensatory elongation of C20:4 to C22:4 as previously described (12). We speculate that the decrease in CE 20:5 relative abundance could be related to a competition between 20:4-CoA and 20:5-CoA as substrates for Acyl-CoA cholesterol acyl transferase (ACAT).
Although there is a controversy regarding the proinflammatory role of LPCAT3 in macrophages (4, 8, 12, 13), we could not find here any evidence for a proinflammatory macrophage phenotype associated with Lpcat3 deficiency either in vitro or in vivo. While this could be due to the partial Lpcat3 deficiency in our model, similar observations were made in fetal liver-derived macrophages with total Lpcat3 deficiency (12). Moreover, in a similar model of myeloid Lpcat3 deficiency, knockout of Lpcat3 in macrophages has no effect on LXR repression of proinflammatory genes, such as Cox2 or Il1b (14).
Transient Lpcat3 inhibition has been associated with ER stress (8); however, we observed here that several markers of ER stress were significantly reduced in Lpcat3KOMac macrophages in basal conditions. Accordingly, Jiang et al. (13) also reported a decrease of the ER stress marker GRP78 (BIP) in Lpcat3-deficient macrophages.
Our group previously reported that Lpcat3−/− macrophages displayed significant alteration of cholesterol homeostasis, including increased free-to-esterified cholesterol ratio and decreased cholesterol efflux (12). We could only partially reproduce this phenotype in the present study, where we observed only a significant reduction of cholesterol efflux while there were no changes in the expression of cholesterol transporters Abca1, Abcg1, or ApoE. We speculate that this is likely related to the residual LPCAT3 activity in Lpcat3KOMac cells. Indeed, it was shown that Lpcat3−/− macrophages presented high levels of nonesterified 22:4 n-6 FA (adrenic acid), a potent LXR antagonist (21). Interestingly the level of this FA was not increased in Lpcat3KOMac cells. Nevertheless, our data suggest that Lpcat3 deficiency may also affect cholesterol efflux by altering cell membrane lipid composition, a hypothesis that deserves future investigations. There was no increase in atherosclerotic lesions in Ldlr−/− mice transplanted with Lpcat3KOMac bone marrow cells. Our data are in accordance with those of Jiang et al. with a similar mouse model (13). In contrast to these observations, transplantation of hematopoietic cells from mice with constitutive Lpcat3 deficiency in Ldlr−/− mice resulted in increased atherosclerotic lesions (12). However, these mice presented a total Lpcat3 deficiency in all the hematopoietic lineages, including hematopoietic stem cells, which is likely to contribute to atherosclerosis development. Indeed, Ldlr−/− mice transplanted with Lpcat3−/− hematopoietic cells presented significantly higher monocyte counts with a relative increase in Ly-6Chigh monocyte subsets, a phenotype that was not observed in the Lpcat3KOMac mouse model (12).
Obesity is another inflammatory chronic disease, in which macrophages play a critical role (22), and is a major risk factor for nonalcoholic fatty liver disease (NAFLD) (23). Here, we found that Lpcat3KOMac mice fed an HFD gained weight with a similar rate as control mice but developed liver metabolic alterations including hepatic steatosis. This was associated with increased liver gluconeogenesis and decreased FA oxidation, but without histological signs of liver inflammation. Transcriptomic profiling of liver myeloid cells did not reveal major differences between Lpcat3KOMac and control mice. This data suggests therefore that partial Lpcat3 deficiency has no major impact on macrophage activation, as observed in vitro, despite changes in AA composition in liver myeloid cells. Accordingly, lipidomic profiling of the whole liver confirmed some alterations in AA homeostasis, including increased concentrations of AA-derived mediators such as TxB2 and LTB4, which are known to promote insulin resistance, inflammation (24, 25), antiplatelet activation (26). While no changes in inflammatory genes were observed, transcriptomic analysis by RNA sequencing shows alterations of cytochrome P450 pathways in the liver of Lpcat3KOMac mice fed an HFD with decrease of Cyp4a12b and increase of Cyp4a14 expression. These genes are notably responsible for the formation of 20-HETE, which is involved in hepatic steatosis (27). Overexpression of Cyp4a14 has been shown to be responsible for increase of FAT/CD36 expression that promotes liver triglyceride content (28). However, we did not find here any difference in 20-HETE liver content or CD36 expression.
While the molecular mechanisms linking hepatic steatosis and myeloid Lpcat3 deficiency remain to be elucidated, our hypothesis is that changes in AA metabolism-restricted liver myeloid cells may secondarily impact AA homeostasis in the whole liver leading to metabolic disorders and TG accumulation. In conclusion, the role of LPCAT3 in controlling macrophage functions seems to be complex. It will deserve future studies with new experimental approaches and models.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors gratefully acknowledge A. Sequiera and S. Monier from the platform of Cytometry; JP Pais de Barros, H. Choubley, and V. Bergas from the Lipidomic platform; A. Chlémaire, A. Bataille, A. Geissler, and A. Bouchot from the platform of Histology; and V. Saint-Giorgio from the Centre de Zootechnie of the Université de Bourgogne for animal care.
Author contributions
T. B. and J. G. conceptualization; T. B. and J. G. methodology; T. B. and J. G. validation; T. B. and J. G. formal analysis; T. B., J. G., D. M., A. J., C. T., J.-P. P. d. B., L. J. L., and T. G. investigation; T. B. and J. G. writing - original draft; T. B. and J. G. visualization; T. B., A. J., C. T., N. L. G., T. G., J.-P. P. d. B., V. B., H. C., L. Mazzeo, L. Menegaut, C. M., L. J. L., K. V. D., M. X., T. J., C. B., J. L., P. S., L. L., D. M. and J. G. writing - review & editing; J. G. supervision; L. L. and J. G. funding acquisition.
Author ORCIDs
Thibaut Bourgeois https://orcid.org/0000-0001-8992-6106
Charles Thomas https://orcid.org/0000-0001-9998-6698
Thomas Gautier https://orcid.org/0000-0003-1119-7382
Jean-Paul Pais de Barros https://orcid.org/0000-0002-5124-2283
Lorène Lebrun https://orcid.org/0000-0002-4486-9723
Tony Jourdan https://orcid.org/0000-0001-7955-8127
Jérome Labbé https://orcid.org/0000-0002-3370-3701
Philippe Saas https://orcid.org/0000-0002-8857-9939
Laurent Lagrost https://orcid.org/0000-0002-8878-6081
David Masson https://orcid.org/0000-0003-1692-0699
Jacques Grober https://orcid.org/0000-0003-0503-4819
Funding and additional information
This research was funded by grants from the Univ. Bourgogne Franche-Comté, the Institut National de la Santé et de la Recherche Médicale (INSERM), by a French Government grant managed by the French National Research Agency under the program “Investissements d'Avenir” with reference ANR-11-LABX-0021 (Lipstic Labex) and by French “Investissements d'Avenir” program, project ISITE-BFC (contract ANR-15-IDEX-0003). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the article, or in the decision to publish the results.
Footnotes
This article contains supplemental data.
Supplemental data
References
- 1.Hishikawa D., Hashidate T., Shimizu T., Shindou H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014;55:799–807. doi: 10.1194/jlr.R046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shindou H., Hishikawa D., Harayama T., Eto M., Shimizu T. Generation of membrane diversity by lysophospholipid acyltransferases. J. Biochem. 2013;154:21–28. doi: 10.1093/jb/mvt048. [DOI] [PubMed] [Google Scholar]
- 3.Harayama T., Eto M., Shindou H., Kita Y., Otsubo E., Hishikawa D., Ishii S., Sakimura K., Mishina M., Shimizu T. Lysophospholipid acyltransferases mediate phosphatidylcholine diversification to achieve the physical properties required in vivo. Cell Metab. 2014;20:295–305. doi: 10.1016/j.cmet.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi M., Varin A., Filomenko R., Lopez T., Athias A., Gambert P., Blache D., Thomas C., Gautier T., Lagrost L., Masson D. Liver X receptor regulates arachidonic acid distribution and eicosanoid release in human macrophages. Arterioscler. Thromb. Vasc. Biol. 2013;33:1171–1179. doi: 10.1161/ATVBAHA.112.300812. [DOI] [PubMed] [Google Scholar]
- 5.Rong X., Wang B., Dunham M.M., Hedde P.N., Wong J.S., Gratton E., Young S.G., Ford A.F., Tontonoz P. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife. 2015;25:4. doi: 10.7554/eLife.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashidate-Yoshida T., Harayama T., Hishikawa D., Morimoto R., Hamano F., Tokuoka S.M., Eto M., Tamura-Nakano M., Yanubo-Takanashi R., Mukumoto Y., Kiyonari H., Okamura T., Kita Y., Shindou H., Shimizu T. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife. 2015;4 doi: 10.7554/eLife.06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A.B., Liu J. Identification of hepatic lysophosphatidylcholine acyltransferase 3 as a novel target gene regulated by peroxisome proliferator-activated receptor δ. J. Biol. Chem. 2017;292:884–897. doi: 10.1074/jbc.M116.743575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong X., Albert C.J., Hong C., Duerr M.A., Chamberlain B.T., Tarling E.J., Ito A., Gao J., Wang B., Edwards A.E., Jung M.E., Ford D.A., Tontonoz P. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z., Jiang H., Ding T., Lou C., Bui H.H., Kuo M.-S., Jiang X.-C. Deficiency in lysophosphatidylcholine acyltransferase 3 reduces plasma levels of lipids by reducing lipid absorption in mice. Gastroenterology. 2015;149:1519–1529. doi: 10.1053/j.gastro.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong X., Wang B., Palladino E.N., de Aguiar Vallim T.Q., Ford D.A., Tontonoz P. ER phospholipid composition modulates lipogenesis during feeding and in obesity. J. Clin. Invest. 2017;127:3640–3651. doi: 10.1172/JCI93616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B., Rong X., Duerr M.A., Hermanson D.J., Hedde P.N., Wong J.S., de Aguiar Vallim T.Q., Cravatt B.F., Gratton E., Ford D.A., Tontonoz P. Intestinal phospholipid remodeling is required for dietary-lipid uptake and survival on a high-fat diet. Cell Metab. 2016;23:492–504. doi: 10.1016/j.cmet.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas C., Jalil A., Magnani C., Ishibashi M., Queré R., Bourgeois T., Bergas V., Ménégaut L., Patoli D., Le Guern N., Labbé J., Gautier T., Pais de Barros J.P., Lagrost L., Masson D. LPCAT3 deficiency in hematopoietic cells alters cholesterol and phospholipid homeostasis and promotes atherosclerosis. Atherosclerosis. 2018;275:409–418. doi: 10.1016/j.atherosclerosis.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H., Li Z., Huan C., Jiang X.-C. Macrophage lysophosphatidylcholine acyltransferase 3 deficiency-mediated inflammation is not sufficient to induce atherosclerosis in a mouse model. Front. Cardiovasc. Med. 2018;5:192. doi: 10.3389/fcvm.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas D.G., Doran A.C., Fotakis P., Westerterp M., Antonson P., Jiang H., Jiang X.-C., Gustafsson J.-A., Tabas I., Tall A.R. LXR suppresses inflammatory gene expression and neutrophil migration through cis-repression and cholesterol efflux. Cell Rep. 26 2018;25:3774–3785.e4. doi: 10.1016/j.celrep.2018.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aparicio-Vergara M., Tencerova M., Morgantini C., Barreby E., Aouadi M. Isolation of Kupffer cells and hepatocytes from a single mouse liver. Methods Mol. Biol. 2017;1639:161–171. doi: 10.1007/978-1-4939-7163-3_16. [DOI] [PubMed] [Google Scholar]
- 16.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 17.Kroetz D.L., Zeldin D.C. Cytochrome P450 pathways of arachidonic acid metabolism. Curr. Opin. Lipidol. 2002;13:273–283. doi: 10.1097/00041433-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Linton M.F., Fazio S. Macrophages, inflammation, and atherosclerosis. Int. J. Obes. 2003;27:S35–S40. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- 19.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi J., Hua L., Harmer D., Li P., Ren G. Cre driver mice targeting macrophages. Methods Mol. Biol. 2018;1784:263–275. doi: 10.1007/978-1-4939-7837-3_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawar A., Xu J., Jerks E., Mangelsdorf D.J., Jump D.B. Fatty acid regulation of liver X receptors (LXR) and peroxisome proliferator-activated receptor α (PPARα) in HEK293 cells. J. Biol. Chem. 2002;277:39243–39250. doi: 10.1074/jbc.M206170200. [DOI] [PubMed] [Google Scholar]
- 22.Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L., Liu D.-W., Yan H.-Y., Wang Z.-Y., Zhao S.-H., Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17:510–519. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- 24.Li P., Oh D.Y., Bandyopadhyay G., Lagakos W.S., Talukdar S., Osborn O., Johnson A., Chung H., Maris M., Ofrecio J.M., Taguchi S., Lu M., Olefsky J.M. LTB4 causes macrophage–mediated inflammation and directly induces insulin resistance in obesity. Nat. Med. 2015;21:239–247. doi: 10.1038/nm.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei X., Li Q., Rodriguez S., Tan S.Y., Seldin M.M., McLenithan J.C., Jia W., Wong G.W. Thromboxane synthase deficiency improves insulin action and attenuates adipose tissue fibrosis. Am. J. Physiol. Endocrinol. Metab. 2015;308:E792-804. doi: 10.1152/ajpendo.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graziani F., Biasucci L.M., Cialdella P., Liuzzo G., Giubilato S., Della Bona R., Pulcinelli F.M., Iaconelli A., Mingrone G., Crea F. Thromboxane production in morbidly obese subjects. Am. J. Cardiol. 2011;107:1656–1661. doi: 10.1016/j.amjcard.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 27.Gilani A., Pandey V., Garcia V., Agostinucci K., Singh S.P., Schragenheim J., Bellner L., Falck J.R., Paudyal M.P., Capdevila J.H., Abraham N.G., Schwartzman M.L. High-fat diet-induced obesity and insulin resistance in CYP4a14−/− mice is mediated by 20-HETE. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R934–R944. doi: 10.1152/ajpregu.00125.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X., Li S., Zhou Y., Su W., Ruan X., Wang B., Zheng F., Warner M., Gustafsson J.-A., Guan Y. Ablation of cytochrome P450 omega-hydroxylase 4A14 gene attenuates hepatic steatosis and fibrosis. PNAS. 2017;114:3181–3185. doi: 10.1073/pnas.1700172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.