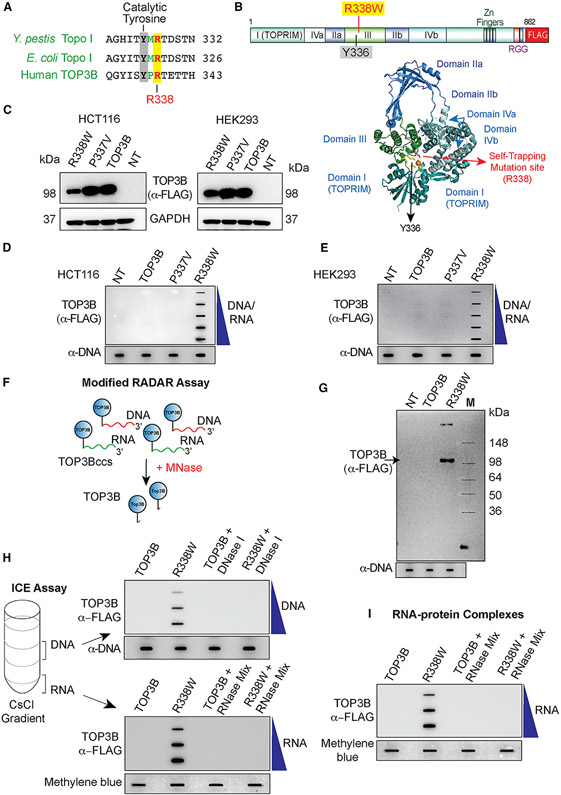
Figure 1. TOP3B Forms TOP3Bccs Both with DNA and RNA in Cells Transfected with R338W-TOP3B.
(A) Alignment of the active site regions of Y. pestis Topo I, E. coli Topo I, and human TOP3B.
(B) Structure of human TOP3B and ribbon representation of human TOP3B (amino acid [aa] residues 1–612) (Goto-Ito et al., 2017) with the active site Y336 and the self-trapping mutation site (R338).
(C) Ectopic expression of wild-type (WT) TOP3B, P337V, and R338W-TOP3B following transfection of HCT116 and HEK293 cells with the indicated TOP3B constructs for 72 h. Western blotting with anti-FLAG antibody.
(D and E) Detection of TOP3Bccs by RADAR assay in cells transfected with the indicated plasmid constructs for 72 h. TOP3Bccs were detected with anti-FLAG antibody. Equal loading was determined by slot blotting and probing with anti-dsDNA antibody. The figure is representative of three independent experiments. NT, mock-transfected cells.
(F) FLAG-tagged TOP3B (blue circles) cellular TOP3Bccs in DNA (red) and RNA (green) were digested with micrococcal nuclease (MNase) followed by SDS-PAGE and immunoblotting with anti-FLAG antibody.
(G) Modified RADAR assay in HEK293 cells transfected with WT or R338W-TOP3B for 72 h. TOP3B was detected with anti-FLAG antibody. Equal loading was tested by slot blotting and probing with anti-dsDNA antibody.
(H) TOP3Bccs in DNA and RNA of HEK293 cells transfected for 72 h with WT or R338W-TOP3B. Cesium chloride gradient ultracentrifugation was performed to separate DNA and RNA (middle and bottom of the gradient, respectively). DNA and RNA fractions were treated with excess RNase A (200 μg/mL) and RNase T1 (200 Units/ml) or DNase I (10 units) as indicated. DNA and RNA fractions were slot blotted and TOP3Bccs detected with anti-FLAG antibody. Equal loading was determined by slot blotting and probing with anti-dsDNA antibody (DNA) or methylene blue staining (RNA). The figure is representative of three independent experiments.
(I) TOP3Bccs in RNA of HEK293 cells transfected with WT or R338W-TOP3B for 72 h. Covalent protein-RNA adducts were isolated using TRIzol (Thermo Scientific) and treated with excess RNase A (200 μg/mL) and RNase T1 (200 units/ml) as indicated. Slot-blotted TOP3Bccs were detected with anti-FLAG antibody. Equal loading was determined by slot blotting and methylene blue staining. The figure is representative of three independent experiments.
See also Figure S1.