Abstract
In addition to causing health concerns, the new coronavirus has been considered in the world with its unknown mechanism of physiopathogenesis and long-term effects after patient recovery. Pulmonary, renal, hepatic and cardiac complications have been reported so far. Beside the researchers' focus on finding vaccines and using conventional therapies, cell-based therapy might be an effective therapeutic strategy. The use of mesenchymal stem cells (MSCs) is one of the options due to their immunomodulatory properties and their proven effects in the treatment of many diseases. As MSCs are not infected with covid-19, there is evidence that it modulates the immune system and prevents the virus from clotting. Despite the beginning of numerous clinical trials in the use of mesenchymal stem cells, it is necessary to set a practical guideline that specifies items such as cell origin, number of cells, frequency of injection, injection site, etc.
Abbreviations: MSC, mesenchymal stem cells; ARDS, acute respiratory distress syndrome; NK, natural killer; DC, dendritic cell; hUCMSC, umbilical cord mesenchymal stem cell; hWJCs, Wharton's jelly-derived MSCs; IDO, indolelamine 2,3-dioxygenase; LIF, leukemia inhibitory factor
Keywords: Mesenchymal stem cells, Covid 19, Treatment, Tissue regeneration
1. Introduction
The new coronavirus pandemic (SARS-CoV2), also known as Covid 19, has had an undeniable impact on people's individual and social lives, overshadowing many social, economic and scientific activities (Niederman et al., 2020, Wu et al., 2020). Researchers in various fields of medicine have made great efforts to find ways to prevent and treat covid 19, which has relatively yielded promising results (Backer et al., 2020, Li et al., 2020). Despite many studies on the effect of control proceedings to reduce the prevalence of the disease, it seems that due to the high emission power of the virus that can keep a person carrier for more than a month, as well as the long hiding time of the disease or the presence of carriers without The symptoms, the complete control of the disease does not make much sense until access to an effective vaccine (Backer et al., 2020, Yi et al., 2020). An important point that has been somewhat neglected is that efforts to reduce the effects of the disease on people with the disease have recovered. Most sufferers may have the effects of covid 19 for years and even the rest of their lives and suffer irreparable damage (Baud et al., 2020, Niederman et al., 2020). There is ample evidence of irreversible pulmonary, cardiac, and renal impairment in recovering patients (Rothan and Byrareddy, 2020). On the other hand, stem cells, in addition to their many applications in the treatment of irreversible disorders of various organs and tissues, play an undeniable role in tissue engineering and production of living tissues (Baud et al., 2020, Metcalfe, 2020). In this article, we will look at the different aspects of using mesenchymal stem cells in the process of healing and tissue regeneration after recovery in patients with covid 19 (Fig. 1 ).
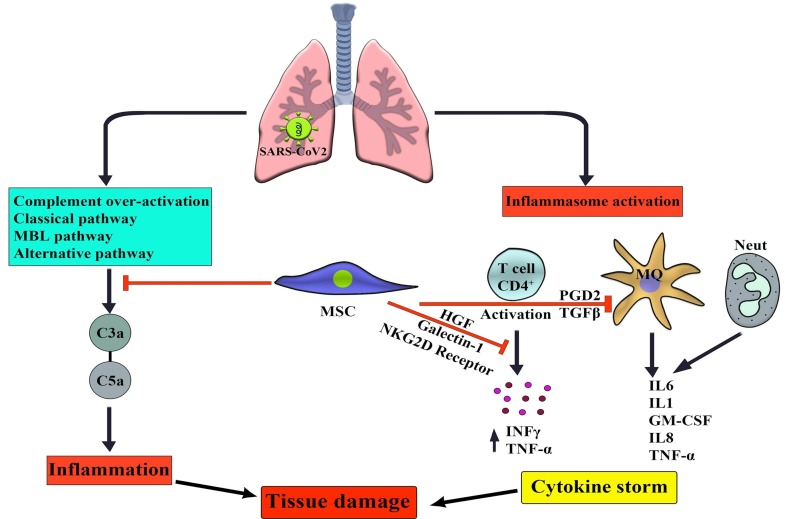
Fig. 1.
Mechanism effect of mesenchymal stem cells on the control of pathological complications of Covid 19. Mesenchymal stem cells, while inhibiting various complement activation pathways, prevent the release of as much C3a/C5a as possible, resulting in inflammation and in continuing tissue damage will be controlled. On the other hand, following infection, the released inflammasomes cause the activation beyond the control of the immune system with the priority of macrophages and neutrophils. Which can control the formation of cytokine storm (IL1/6/TNFα).
2. Covid-19
The RNA virus, a positive-sense single-stranded RNA with a genome length of 30 kb, is a member of the Nidovirus, Coronavirus family, and beta-coronavirus (Wong et al., 2019, Wu et al., 2020). Order: Nidovirales, Family: Coronaviridae, Genus: Betacoronavirus.
Almost all countries in the world are exposed to the coronavirus pandemic and severe economic, social and therapeutic consequences have been palpable to all people of the world (Wong et al., 2019, Niederman et al., 2020). Therapeutic Approaches in terms of treatment, vaccine production has not been very successful, and many researchers have focused their research on patients with clinical residual effects after getting Corona disease so that they may be able to better cope with and manage disease complications (Backer et al., 2020, Ji et al., 2020, Prompetchara et al., 2020). There is ample clinical evidence that covid 19 is a multifaceted disease involving the body and various organs such as the heart, lungs, kidneys, muscles, and central nervous system (Baud et al., 2020, Rothan and Byrareddy, 2020). However, there are also ambiguous conflicts between the coagulation system and homeostasis and the skin that need further investigation (Porfidia and Pola, 2020). For this reason, the treatments used have low efficacy and many side effects until the exact mechanism of pathogenesis of the disease is discovered (Hamming et al., 2004, Rothan and Byrareddy, 2020). Despite the contradictory evidence, therapies change over time and are replaced by better and more effective drugs (Prompetchara et al., 2020). Recently, many authoritative studies with significant sample size have ruled out the use of chloroquine and its derivatives, which were previously considered the forerunner of treatment, and recommended the use of conventional antiviral drugs for Ebola and AIDS such as Ramsesvir and Favipiravir (Wang et al., 2020). The use of antibiotics that allow for viral control and secondary bacterial infection can be as effective as anti-inflammatory drugs that control the cytokine storm (Ye et al., 2020). The process, which causes inflammatory and pre-inflammatory cytokines such as interleukins 6, 7, 2, and 1, as well as GSCF, MCP1, and TNFα, causes inflammation and pulmonary edema, impaired gas exchange, and ultimately acute respiratory distress syndrome (ARDS) (Leng et al., 2020, Mehta et al., 2020, Prompetchara et al., 2020). Although the process is blamed for acute heart and other organ damage that eventually ends in the patient's death, recent evidence suggests that microthrombosis occurs in the capillaries and arteries of various organs, with increasing evidence of myalgia and heart and brain stroke can also reinforce this hypothesis (Prompetchara et al., 2020, Zhang et al., 2020).
3. Mesenchymal stem cells
Adult stem cells, the most important of which are mesenchymal stem cells, in addition to their specific use in the treatment of tissue lesions of mesenchymal origin such as bone, cartilage, muscle, liver, tendon and adipose tissue, due to the protective role of the cell blood vessels in the bone marrow, as well as their undeniable role in immunomodulation, are highly important in regulating the immune system (Kehtari et al., 2019, Ji et al., 2020). The treatment of many inflammatory diseases, especially autoimmune diseases such as multiple sclerosis, lupus, rheumatoid arthritis, etc., has been investigated using mesenchymal stem cells (Chen et al., 2020). There is even evidence of the effect of mesenchymal stem cells in the treatment of influenza H7N9, which apparently has similar physiopathogenesis. The control process of the immune system by MSC has been proven by controlling T, B, natural killer (NK) lymphocytes and dendritic cells (DC) activated by the secretion of anti-inflammatory cytokines such as IL10, IL4, etc (Weiss and Dahlke, 2019).
There is evidence that mesenchymal stem cells stop the activation of macrophages by reducing the expression of MHC-II, CD11, and CD83, and reduce the secretion of pre-inflammatory cytokines such as TNFα and IL12. Stopping T lymphocytes in the G0/G1 phase of the cell cycle and also reducing the secretion of TNFα, IL6 and IL17 pre-inflammatory cytokines are other effects of MSC on the human immune system (Weiss and Dahlke, 2019, Golchin et al., 2020).
However, it can be said that the greatest effect of mesenchymal stem cells is to help regulate and regulate the immune system on each side of its hyperactivity (Glenn and Whartenby, 2014). Despite the abundant evidence of Th1 suppression and the reduction in INFϒ secretion, there is evidence that in allergic lung inflammation, which is associated with increased Th2 function, MSCs stimulate INFϒ secretion and lead to a Th1 immune response (Glenn and Whartenby, 2014, Golchin et al., 2020). Other evidence of this dual role is the modification of Th17 and Treg, which are different evidence of control of these cells by controlling the secretion of their cytokines (Golchin et al., 2020). The sum of these findings suggests that mesenchymal stem cells while secreting anti-inflammatory agents, maybe a good barrier to the formation of cytokine storms and may also repair previous lesions.
Clinical efficacy studies using MSC in patients with acute corona phase, performed by various researchers in the early days of corona prevalence, also confirmed these laboratory findings (Gorman et al., 2020, Leng et al., 2020, Zhang et al., 2020). The patient connected to the ventilator with a hepatic complication following the injection of three turns of umbilical cord mesenchymal stem cell (hUCMSC = 5 × 107) underwent a rapid recovery process and was discharged from the hospital without any side effects (Leng et al., 2020). A similar experiment was repeated in 7 covid patients (severe to moderate form), all suffering from severe clinical symptoms of corona (low oxygen saturation and high fever), and intravenous MSC injection (1 × 106 per Kg) discharged hospitalized patients quickly. Laboratory evidence of decreased inflammation and pre-inflammatory cytokines and increased immune system control was associated with increased IL10. In another experiment, which was performed by injecting human umbilical cord Wharton's jelly-derived MSCs (hWJCs) from a healthy donor to a person with corona virus under alarming conditions, the improvement resulted in clearance of the patient after one week, while the number of lymphocytes T increased in his blood and the inflammatory indices (IL-6, TNF-α, and C-reactive protein) decreased significantly (Zhang et al., 2020). It can be said that the migration of MSCs after injection into the organs involved in inflammation, especially the lungs, has led to infection control and even repair of fibrous tissue lesions. The same mechanism can be used in liver, heart, kidney, etc. Lesions that may be caused by a coronavirus infection. Noting that targeted treatment with this precision could take months and not be as effective. Similar studies have shown that injection of cord MSCs (5x107) compensated for lymphopenia (increased CD4+ Tcell and CD8+ Tcell) caused by Covid 19 and greatly compensated for tissue damage factors such as ALT/AST and increased CRP (Zhang et al., 2020). Significant reductions in CD8 and CD4+ lymphocytes with CXCR3 +, as well as a decrease in TNFα levels and an increase in IL10 levels, have been reported as promising findings in the treatment of patients with Covid 19 with MSC in limited studies (Zhang et al., 2020).
There is also evidence that MSCs, in addition to having an indirect effect on inflammation by stimulating the production of antimicrobial proteins (AMP) and indolelamine 2,3-dioxygenase (IDO), also having a direct effect on the activation of macrophages through innate immunity makes us see effective applications of MSC in sepsis, ARDS, and cystic fibrosis infection (Vasandan et al., 2016, Metcalfe, 2020). It also seems that despite the low specificity of intrinsic immunity, fewer side effects of this system are effective in clearing the virus in the body. This process can lead to less inflammation by inducing damage to the integrity of the lipid membrane of the virus or inhibiting its reproductive mechanisms and intracellular overlap. The human immune system also helps with this process by increasing the expression of various acute phase proteins such as CRP, ferritin, etc (Zheng et al., 2018, Schijns and Lavelle, 2020). The altered mesenchymal stem cells or derivatives of these cells, such as the supernatant of their culture medium, which themselves may contain large amounts of anti-inflammatory factors, may have similar effects (Zheng et al., 2018).
Additional aspects of physiopathogenesis in coronavirus include thrombotic and coagulation complications (Chi et al., 2020, Spyropoulos et al., 2020). There are many studies that suggest that the physiopathology of the disease may be due to capillary microthrombosis in various organs (Fogarty et al., 2020, Tang et al., 2020). Other evidence for this claim includes an increase in the incidence of myocardial infarction in medical centers for patients with corona virus (Iba et al., 2020). This finding is even more valuable when we consider that most cases of heart attack and stroke occur in young people, and of course the response to thromboprophylaxis treatments such as influenza H1N1 has been promising (Spyropoulos et al., 2020). There is also ample evidence of an increase in D-Dimer in the bloodstream of patients and the occurrence of DIC in various studies (Marchandot et al., 2020). It has also been shown that abnormal coagulation parameters have a significant relationship with poor disease prognosis. In addition to exacerbating the effects of the cytokine storm, it seems that inhibiting adequate blood supply to tissues, especially the lungs, can reduce the percentage of oxygen saturation in the blood and cause severe complications (Spyropoulos et al., 2020). This finding is confirmed by the presence of the surface antigen of hemagglutinin on the surface of viruses so that the surface antigen of hemagglutinin strastase virus in addition to effective in binding the virus to the host cell can intensify the process of induction of microconductivity through this receptor (Avti et al., 2020). One of the applications of stem cells that have been considered so far is its widespread use in cases of schematic tissue events. One of the most important of these events is stroke, which has been targeted by various types of stem cells, especially mesenchymal stem cells (Toyoshima and Yasuhara, 2017). Mesenchymal stem cells, in addition to their immunomodulatory effects, contribute significantly to the regeneration of neurons and synaptogenesis. Some studies have also noted the anticoagulant effects of mesenchymal stem cells (Massoud et al., 2014).
Other evidence suggests that the lethality of covid 19 is related to serum levels of vitamin D, and that many findings suggest that vitamin D is an effective anticoagulant that will increase the risk of microthrombosis (García-Carrasco et al., 2018, McCartney and Byrne, 2020).
Mesenchymal stem cells have many uses in achieving this goal, but the use of different sources to obtain them, such as umbilical cord, adipose tissue, and bone marrow, is not well agreed (Mirzaei et al., 2019, Rogers et al., 2020). Despite the limitations of bone marrow and adipose tissue sampling, as well as the small amount and speed of the mesenchymal cells extracted from it, umbilical cord cells have a higher priority.
Another interesting point is that in order to transfer MSC, it can be done in different ways, but there is evidence that shows that one of the centers of concentration of MSC is after intravenous injection of the lungs, although different organs show the effects of this injection, which can be caused by MSC secretions such as exosomes and anti-inflammatory cytokines (Khatri et al., 2018, Zheng et al., 2018).
4. Cell therapy during Covid 19 pandemic
In addition to most Elective treatments, such as cosmetic surgery, many essential treatments are stopped or delayed during coronavirus pandemic (Ji et al., 2020, Orleans and is Vice, H. and Manchikanti, L., , 2020). Most cases are based on the patient's request for general anxiety conditions in the community, and also the role of incorrect information and the induction of excessive fear by the media cannot be ignored. Many patients with acute illnesses, such as those with blood malignancies that require immediate bone marrow transplantation; have voluntarily discontinued their treatment, which can lead to irreversible complications.
Interestingly, the study of mesenchymal cells indicates that MSC is not contaminated with Covid 19. There is evidence that ACEII receptors, which play the role of virus receptors on MSC cells, either do not exist or are expressed to a minimal extent. In contrast, the expression of TMPRSS2 as an internal factor in the initial pathogenesis of the virus has little expression in MSCs (Hamming et al., 2004, Hoffmann et al., 2020, Leng et al., 2020). This process was confirmed by the RNA-Seq method during MSC transplantation, which can relieve recipients of mesenchymal stem cell transplantation from MSC virus transmission. In addition, these same cells, with their pronounced profile of long-acting anti-inflammatory and trophic cytokines (TGFb, LIF, and VEGF), induce effective immunomodulatory properties (Quinton et al., 2012, Foronjy et al., 2014). The Leukemia Inhibitory Factor (LIF) itself is an effective factor in combating cytokine storms that can make the use of MSC more effective.
However, it seems that interruption in the process of essential cell therapy, especially HCT, in patients with different types of leukemia can, in addition to irreparable physical damage, further degrade a person's immune system and lead to severe disease.
Although it is recommended that non-emergencies be delayed for several months, special emergency centers can be set up that, in addition to carefully examining the people being treated for infections, provide a few days pre-hospitalization to ensure they are not transported and are maintained for Long-term after transplantation (Dholaria and Savani, 2020).
5. Discussion
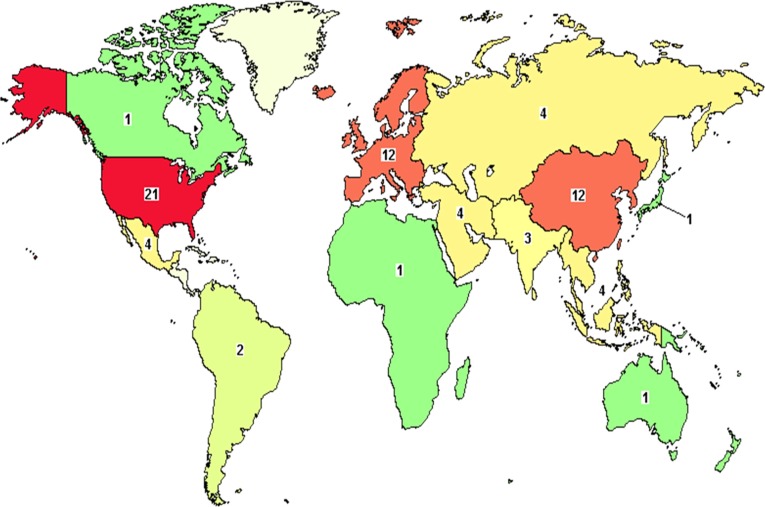
The unknown physiopathological mechanism of Covid 19, such as the multifaceted involvement of the immune system and the occurrence of cytokine storms, the simultaneous involvement of multiple organs, can enhance the use of stem cells (Metcalfe, 2020, Prompetchara et al., 2020). On the other hand, the time-consuming process of vaccine production and the lack of a clear vision for effective drug delivery reinforce the suspicion that the vaccine and effective drug are likely to be obtained when either the pandemic has been self-limiting or the vast majority of the world's population has been diagnosed with the disease (Prompetchara et al., 2020, Yi et al., 2020). This approach further highlights the need to think about treatment and reduce the complications of the disease. One of the new therapies that have few side effects is cell therapy based on mesenchymal stem cells. Cells can be extracted from various tissue sources such as adipose tissue, peripheral blood, bone marrow, Wharton gel, amniotic fluid, dental pulp, umbilical cord blood, etc (Chen et al., 2020, Golchin et al., 2020). As of this article is being written, there are over 44 research projects reviewing the impact of cell therapy or related products on the treatment of Covid 19 at the American Clinical Trials Research Center (https://clinicaltrials.gov/) which has been reported that they are often sampling and performing in several centers. By the time of final review of the article (December 24, 2020), 81 clinical trial articles using stem cells on Covid-19 have been registered in the American Clinical Trial Database (ClinicalTrial.gov), of which only 7 have been completed and the rest still running (Fig. 2 , Table 1 ). The United States with 21 studies and China as the European countries with 12 studies have been the most active in this field. Also, umbilical cord mesenchymal derived stem cells, bone marrow derived stem cells and exosomes isolated from mesenchymal stem cells have been the most therapeutic targets.
Fig. 2.
Global distribution of clinical trial studies using stem cells on Covid-19 (12/24/2020 – ClinicalTrials.gov).
Table 1.
Clinical trial completed project about Covid-19 (12/24/2020 - ClinicalTrials.gov).
| ClinicalTrials.gov ID: | Phase | participants | region of cell | center (country) | Allocation Model | Intervention |
|---|---|---|---|---|---|---|
| NCT04288102 | II | 100 | Human Umbilical Cord-derived Mesenchymal Stem Cells | Beijing 302 Hospital (China) | Randomized, double-blind, placebo-controlled study (Parallel Assignment) | 3 does of UC-MSCs (4x107 cells per time) intravenously at Day 0, Day 3, Day 6 |
| NCT04473170 | I/II | 146 | Autologous Non-Hematopoietic Peripheral Blood Stem Cells (NHPBSC) | Abu Dhabi Stem Cells Center (UAE) | Randomized (Parallel Assignment) | N/A |
| NCT04355728 | I/II | 24 | Human Umbilical Cord-derived Mesenchymal Stem Cells | University of Miami (US) | Randomized, double-blind, placebo-controlled study (Parallel Assignment) | UC-MSC will be administered at 100x106 cells/infusion administered intravenously |
| NCT04573270 | I | 40 | Human Umbilical Cord-derived Mesenchymal Stem Cells | Thomas Advanced Medical LLC (US) | randomized, double-blind, placebo-controlled, multi-arm, multi-site (Single Group Assignment) | Single intravenous injections of mesenchymal stem cells derived from human umbilical chords |
| NCT04492501 | Not Applicable | 600 | Bone Marrow MSC | UNICEF (Pakistan) | Non-Randomized, open label (Factorial Assignment) | Single Dose of 2x106 cells/kg |
| NCT04276987 | I | 24 | MSCs-derived exosomes | Ruijin Hospital (China) | N/A (Single Group Assignment) | 5 times aerosol inhalation of MSCs-derived exosomes (2x108 nano vesicles/3 ml at Day 1, Day 2, Day 3, Day 4, Day 5). |
| NCT04491240 | I/II | 30 | MSCs-derived exosomes | Samara Regional Medical Center Dinasty (Russia) | Randomized, double-blind, placebo-controlled study (Parallel Assignment) | Twice a day during 10 days inhalation of 3 ml special solution contained 0.5-2x1010 of nanoparticles (exosomes) |
Also, in the local governmental database of Clinical Trial of Iran (https://www.irct.ir/) so far (December 24, 2020) 12 studies aimed at using stem cells in the treatment of Covid-19 have been recorded, all of which are still in are running.
However, one of the constant concerns during the clinical use of stem cells is their chances of tumor formation, which has led to more caution in this area. In addition to production under the necessary controls (cGMP) is another necessity that should not be overlooked due to the acceleration of the production process (Gentile and Sterodimas, 2020, Orleans and is Vice, H. and Manchikanti, L., , 2020). It is important for donors and recipients to be identified that it signifies that strict criteria are unavoidable. The dose of the injection, the best way of cellular transmission, the amount and concentration of the infusion should all be clearly confirmed (Shetty, 2020). Ensuring that the donor does not become infected with Covid 19 is as important as many other viral infections, such as Epstein-Barr virus and cytomegalovirus.
Precautions should be taken when injecting in a hospital, especially at risks such as emboli, septicemia, and allergies. Monitoring and follow-up of the patient for at least three weeks are recommended. At this time, in addition to examining the factors associated with Covid 19 infection, tissue and immunological evaluation should also be considered. Although there is evidence of supportive effects of MSC in the treatment of Covid 19 (Chen et al., 2020, Gentile and Sterodimas, 2020, Golchin et al., 2020), there are no unpleasant side effects, but due to the high risk of most people in the acute phase Disease (suppressed immune system, high blood pressure, history of heart complications, etc.), more comprehensive studies appear to be needed to make a decision.
6. Authors’ contribution
E.S., MF.A., H.H., R.NM., R.E., M.G., M.R., SE.E. conceptualized the study, drafting, writing the manuscript, and approving it for submission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported by research grants from the Mazandaran University of Medical Sciences, Sari, Iran (project number: 8177).
References
- Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya A., Kumar H., Bansal S., Medhi B. Drug targets for corona virus: A systematic review. Indian J. Pharmacol. 2020;52 doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Eurosurveillance. 2020;25:2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. LancetInfect. Dis. 2020 doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X., Chen L., Gao H., Lu X., Yu L. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering. 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi G., Lee J.J., Jamil A., Gunnam V., Najafi H., Memar Montazerin S., Shojaei F., Marszalek J. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. Journal of clinical medicine. 2020;9:2489. doi: 10.3390/jcm9082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dholaria B., Savani B.N. How do we plan hematopoietic cell transplant and cellular therapy with the looming COVID-19 threat? Br. J. Haematol. 2020;189:239. doi: 10.1111/bjh.16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020 doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy R.F., Dabo A.J., Cummins N., Geraghty P. Leukemia inhibitory factor protects the lung during respiratory syncytial viral infection. BMC immunology. 2014;15:41. doi: 10.1186/s12865-014-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carrasco M., Jiménez-Herrera E., Gálvez-Romero J., Mendoza-Pinto C., Méndez-Martínez S., Etchegaray-Morales I., Munguía-Realpozo P., Vázquez de Lara-Cisneros L., Santa Cruz F., Cervera R. The anti-thrombotic effects of vitamin D and their possible relationship with antiphospholipid syndrome. Lupus. 2018;27:2181–2189. doi: 10.1177/0961203318801520. [DOI] [PubMed] [Google Scholar]
- Gentile P., Sterodimas A. Adipose stem cells (ASCs) and stromal vascular fraction (SVF) as a potential therapy in combating (COVID-19)-Disease. Aging and disease. 2020;11:465. doi: 10.14336/AD.2020.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J.D., Whartenby K.A. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J. Stem Cells. 2014;6:526. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev. Rep. 2020:1–7. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman E., Shankar-Hari M., Hopkins P., Tunnicliffe W.S., Perkins G.D., Silversides J., McGuigan P., Jackson C., Boyle R., McFerran J. Repair of acute respiratory distress syndrome by stromal cell administration in COVID-19 (REALIST-COVID-19): A structured summary of a study protocol for a randomised, controlled trial. Trials. 2020;21:1–3. doi: 10.1186/s13063-020-04416-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M., Lely A., Navis G.V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of coronavirus disease 2019. Crit. Care Med. 2020 doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Ma Z., Peppelenbosch M.P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Global Health. 2020;8 doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehtari M., Beiki B., Zeynali B., Hosseini F.S., Soleimanifar F., Kaabi M., Soleimani M., Enderami S.E., Kabiri M., Mahboudi H. Decellularized Wharton's jelly extracellular matrix as a promising scaffold for promoting hepatic differentiation of human induced pluripotent stem cells. J. Cell. Biochem. 2019;120:6683–6697. doi: 10.1002/jcb.27965. [DOI] [PubMed] [Google Scholar]
- Khatri M., Richardson L.A., Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018;9:1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Disease. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-R., Tang Z.-J., Li Z.-H., Liu X. Searching therapeutic strategy of new coronavirus pneumonia from angiotensin-converting enzyme 2: the target of COVID-19 and SARS-CoV. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1021. doi: 10.1007/s10096-020-03883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchandot B., Sattler L., Jesel L., Matsushita K., Schini-Kerth V., Grunebaum L., Morel O. COVID-19 related coagulopathy: A distinct entity? J. Clin. Med. 2020;9:1651. doi: 10.3390/jcm9061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud M.R., William B.M., Harrill K., Cooper B.W., Lima M.D., Schmaier A.H. Transmission of lupus anticoagulant by allogeneic stem cell transplantation. Revista brasileira de hematologia e hemoterapia. 2014;36:287–289. doi: 10.1016/j.bjhh.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney D.M., Byrne D. Optimisation of vitamin D status for enhanced Immuno-protection against Covid-19. Ir. Med. J. 2020;113:58. [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Collaboration H.A.S. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe, S.M., 2020. Mesenchymal stem cells and management of COVID-19 pneumonia. Medicine in drug discovery, 100019. [DOI] [PMC free article] [PubMed]
- Mirzaei A., Moghadam A.S., Abazari M.F., Nejati F., Torabinejad S., Kaabi M., Enderami S.E., Ardeshirylajimi A., Darvish M., Soleimanifar F. Comparison of osteogenic differentiation potential of induced pluripotent stem cells on 2D and 3D polyvinylidene fluoride scaffolds. J. Cell. Physiol. 2019;234:17854–17862. doi: 10.1002/jcp.28415. [DOI] [PubMed] [Google Scholar]
- Niederman M.S., Richeldi L., Chotirmall S.H., Bai C. American Thoracic Society; 2020. Rising to the Challenge of COVID-19: Advice for Pulmonary and Critical Care and an Agenda for Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orleans L., is Vice, H. and Manchikanti, L., Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Phys. 2020;23:E71–E83. [PubMed] [Google Scholar]
- Porfidia A., Pola R. Venous thromboembolism in COVID-19 patients. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Quinton L.J., Mizgerd J.P., Hilliard K.L., Jones M.R., Kwon C.Y., Allen E. Leukemia inhibitory factor signaling is required for lung protection during pneumonia. J. Immunol. 2012;188:6300–6308. doi: 10.4049/jimmunol.1200256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C.J., Harman R.J., Bunnell B.A., Schreiber M.A., Xiang C., Wang F.-S., Santidrian A.F., Minev B.R. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J. Transl. Med. 2020;18:1–19. doi: 10.1186/s12967-020-02380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;102433 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijns V., Lavelle E.C. Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity. Eur. J. Immunol. 2020 doi: 10.1002/eji.202048693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty A.K. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging and disease. 2020;11:462. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos A.C., Ageno W., Barnathan E.S. Hospital-based use of thromboprophylaxis in patients with COVID-19. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima A., Yasuhara T. Mesenchymal stem cell therapy for ischemic stroke. Acta Med. Okayama. 2017;71:263–268. doi: 10.18926/AMO/55302. [DOI] [PubMed] [Google Scholar]
- Vasandan A.B., Jahnavi S., Shashank C., Prasad P., Kumar A., Prasanna S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE 2-dependent mechanism. Sci. Rep. 2016;6:38308. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A.R.R., Dahlke M.H. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019;10:1191. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.C., Li X., Lau S.K., Woo P.C. Global epidemiology of bat coronaviruses. Viruses. 2019;11:174. doi: 10.3390/v11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y., Lagniton P.N., Ye S., Li E., Xu R.-H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16:1753. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ding J., Ren S., Wang W., Yang Y., Li S., Meng M., Wu T., Liu D., Tian S. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020;11:1–6. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.H., Deng Y.Y., Lai W., Zheng S.Y., Bian H.N., Liu Z.A., Huang Z.F., Sun C.W., Li H.H., Luo H.M. Effect of bone marrow mesenchymal stem cells on the polarization of macrophages. Mol. Med. Rep. 2018;17:4449–4459. doi: 10.3892/mmr.2018.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]