To the editor,
SARS-CoV-2 has been associated with an increased prevalence of stroke; one suggested pathophysiological mechanism is related to an endotheliopathy of cerebral vessels.1 However, no evidence of such mechanism has been reported in vivo in patients with SARS-CoV-2.
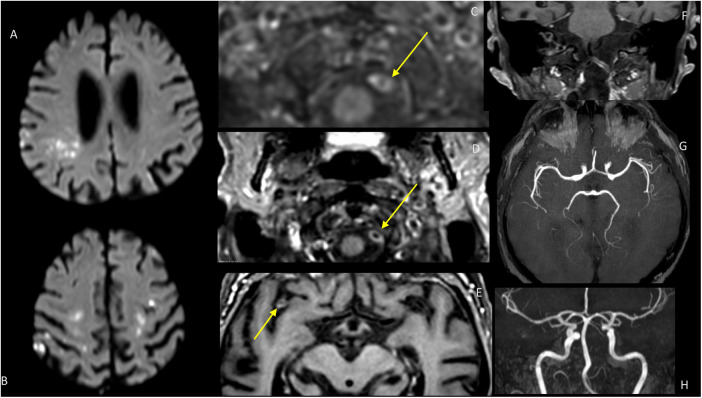
We report the case of a 81-year old patient who presented during a severe SARS-CoV-2 infection multiple cerebral ischemic lesions (Fig. 1 A,B), with contrast enhancement of bilateral vertebral, right carotid and middle cerebral arteries. The patient was known for a single episode of atrial fibrillation treated by apixaban (not present at the time of admission), without any other cardiovascular risk factors.
Fig. 1.
Magnetic resonance imaging of the patient: diffusion-weighted images show hyperintensities in both hemispheres (A, B). Contrast-enhanced vessel wall imaging (C,D,E,F)shows enhancement of the vertebral arteries (C;D,F) and of the right MCA (E). the TOF MRA show no stenosis or occlusion (G,H).
He was admitted November 11, 2020 to our institution for fever, dry cough and dyspnea. Chest CT showed ground-glass opacifications with light pleural effusion. The diagnosis of SARS-CoV-2 was ultimately confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) assay on a nasal swab. During the following days, the patient complained of gait instability and loss of coordination of his left hand. Neurological examination revealed a motoric and ataxic left hemisyndrome, with a NIH stroke scale (NIHSS) of 4. Brain MRI (1.5 T Philips Ingenia) showed multiple small acute and subacute ischemic lesions, in particular in internal border zones between the deep and superficial arterial system of the middle cerebral artery, bilateral posterior cerebral arteries and bilateral cerebellar arteries. In addition, dedicated MR wall imaging (T1-FS contrast- enhanced VISTA) highlighted bilateral vertebral, right carotid and middle cerebral artery gadolinium enhancement. Gradient-echo MRI showed a single frontal microbleed restricted to the cortical regions, compatible with a possible cerebral amyloid angiopathy according to the Boston diagnostic criteria, however we cannot exclude SARS-CoV-2 related microbleeds.2
Due to the distribution of the stroke and the absence of necks vessels stenosis, anticoagulation treatment was maintained, with a switch to low molecular weight heparin. Blood work showed an inflammatory syndrome with an elevated C-reactive protein (100 mg/L), fibrinogen (6.9 g/l) and coagulation activation with lightly elevated d-dimer (716 ng/mL). Platelets count was normal and antiphospholipid antibodies were negative. Transthoracic echocardiography did not detect any cardiac source of embolism.
Because of a deteriorating respiratory status with higher amounts of oxygen needed, the patient was transferred to the intermediate care unit for an acute respiratory distress syndrome and the patient unfortunately died a few days later from a cardiac arrest. Autopsy was denied by the family.
Acute cerebrovascular disease is a frequent manifestation of systemic COVID-19 disease.3, 4, 5, 6, 7, 8 Although the mechanism of injury remains hypothetical, evidence of a SARS-CoV-2 mediated endotheliopathy is emerging.8 Our case supports the hypothesis of cerebral COVID-19-associated endotheliopathy leading to stroke. In our patient, neuroimaging findings show diffuse multi territorial and border zones ischemic lesions, despite the absence of vessels neck stenosis or evidence of decreased arterial pressure (Figure A,B). Brain MRI with angiographic and intracranial vessel wall sequence (TSE FAT SAT VISTA sequence) show bilateral vertebral (Figure C), right carotid and middle cerebral artery intracranial vessel wall contrast enhancement (Figure D). The TOF angiography showed no stenosis that could be present in arteriosclerosis or localized vasculitis, conditions that may occur in older patients but their neuroradiological presentations are different morphologically.9 Due to the morphology of the contrast enhancement, vasa vasorum enhancement was also judged unlikely.10 Consistent with recent neuroimaging and neuropathological studies, diffuse endothelial inflammation in multiple organs, including the central nervous system has been demonstrated in patients infected by SARS-CoV-2. 5, 6, 8 A recent report described first angio-MRI signs of inflammation in CNS arteries consistent with endotheliitis in 5 consecutive patients intubated because of COVID-19 related acute respiratory distress syndrome.3 In a case series of 32 critically ill patients with COVID19 and concomitant severe CNS involvement, Keller et al. conclude that vessels wall enhancement may be the result of an endothelial dysfunction induced by systemic COVID-19 infection.5 Another study showed vessel wall enhancement with a different pattern than in our case.11 A mechanism of direct virus- or/and indirect immune-mediated endothelial injury is considered.5 SARS-CoV-2 uses a spike protein, the angiotensin converting enzyme 2 (ACE2) receptor to infect the host. ACE2 receptors are expressed ubiquitously in organs and tissues, among which endothelial cells.6 Hence, our findings are supportive of an inflammation of cerebral arteries consistent with endothelial dysfunction leading to stroke. Additionally, even if vasa vasorum enhancement cannot be formally excluded, the morphology observed in our case is quite different: it is circumferential and not nodular; also arteriosclerotic enhancement is not usually circumferential and our case has no stenosis on TOF MRA.
The association of ischemic lesions in the same vascular territory is suggestive of an inflammatory vascular wall pathology that may participate to the mechanisms of COVID-19 cerebrovascular disorders. Hanafi et al. described the case of a COVID-19 patient with diffuse periventricular ischemic lesions and postcontrast enhancement pattern consistent with small vessel involvement.4 Hypoxic-ischemic injury and a pro-thrombotic state was not considered a likely causative etiology and because of a characteristic combined imaging pattern of ischemia, hemorrhage and punctuate contrast enhancement, a SARS-CoV-2 induced endotheliitis was retained.
In conclusion, this case with an endotheliopathy suggestive an endotheliitis with multiple stroke supports the hypothesis of an inflammatory pathophysiological mechanism of brain vessels associated with stroke in COVID-19 patients. Future neuroimaging studies should include a systematic evaluation of brain vessel wall imaging in COVID-19 patients with stroke.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitsiori A., Pugin D., Thieffry C., Lalive P., Vargas M.I. COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J Neuroimaging. 2020;30(5):593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugin D., Vargas M.I., Thieffry C., et al. COVID-19-related encephalopathy responsive to high-dose glucocorticoids. Neurology. 2020;95(12):543–546. doi: 10.1212/WNL.0000000000010354. [DOI] [PubMed] [Google Scholar]
- 4.Hanafi R., Roger P.A., Perin B., et al. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am J Neuroradiol. 2020;41(8):1384–1387. doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller E., Brandi G., Winklhofer S., et al. Large and small cerebral vessel involvement in severe COVID-19: Detailed clinical workup of a case series. Stroke. 2020;51(12):3719–3722. doi: 10.1161/STROKEAHA.120.031224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-Fernandez F., Sandoval Valencia H., Barbella-Aponte R.A., et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143(10):3089–3103. doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell D.M., Mossa-Basha M., Qiao Y., et al. Intracranial vessel wall MRI: Principles and expert consensus recommendations of the american society of neuroradiology. AJNR Am J Neuroradiol. 2017;38(2):218–229. doi: 10.3174/ajnr.A4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portanova A., Hakakian N., Mikulis D.J., Virmani R., Abdalla W.M., Wasserman B.A. Intracranial vasa vasorum: insights and implications for imaging. Radiology. 2013;267(3):667–679. doi: 10.1148/radiol.13112310. [DOI] [PubMed] [Google Scholar]
- 11.Lersy F., Anheim M., Willaume T., et al. Cerebral vasculitis of medium-sized vessels as a possible mechanism of brain damage in COVID-19 patients. J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]