Figure 3. The Two Phases of Wound Repair in the Skin.
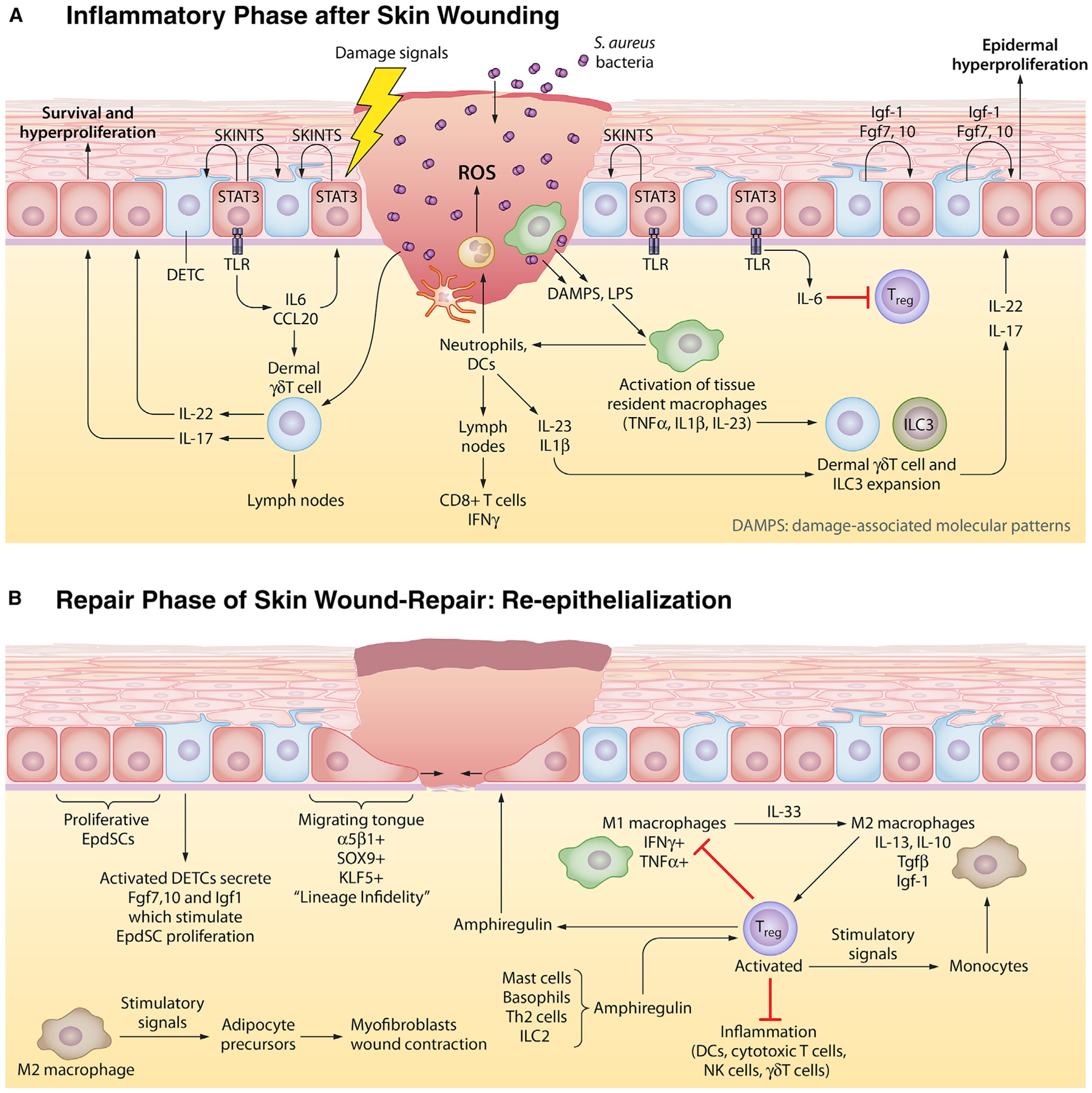
In the skin, the stem cell niche closest to the site of the wound is mobilized to respond. Here, we schematize the response to full-thickness skin wounds, where EpdSCs adjacent to the wound site become mobilized. Wound responses involve two steps. In the first phase, a blood clot forms to seal off the wound and an inflammatory response is triggered to guard against microbial invasion and to clear out damaged cells. In the second phase, inflammation must be dampened to permit re-epithelialization of the damaged skin barrier.
(A) Epithelial inflammatory phase. Damage-associated molecular patterns (DAMPs) and reactive oxygen species (ROS) emanating from wound-damaged tissue and from pathogens, such as Staphylococcus aureus, signal to circulating neutrophils and to resident macrophages to become activated to form so-called M1 macrophages and begin the inflammatory response. Activated antigen-presenting dendritic cells (DCs) migrate to the lymph nodes to activate and recruit cytotoxic T cells to the skin. EpdSCs are not silent during this time. Those adjacent to the wound edge produce SKINTs, factors that mobilize resident DETCs to produce factors that in turn fuel epidermal hyperproliferation next to the wound site. Nearby epidermal cells phosphorylate and activate STAT3, which in turn stimulate production of interleukins and chemokines that stimulate dermal δγ T cells which have been purported to be able to transit to lymph nodes like DCs do. The outcome from this heightened immune response is a plethora of cytokines that further stimulate other immune cells, including innate lymphoid cells (ILCs) to mobilize into action as shown here.
(B) Epithelial repair phase. While inflammation is essential to fight infections, it is incompatible with the re-epithelialization process. For this to occur, inflammation must be dampened. Exactly how is still not clear, but it is known that the expansion of resident regulatory T cells (Tregs) is key. Tregs respond to a number of factors, including amphiregulin, generated by a number of inflammatory cells. Tregs both dampen/exhaust cytotoxic T cell activity and also stimulate the conversion of inflammatory M1 macrophages to repair M2 macrophages. M2 macrophages have multiple functions, one of which is to produce factors such as TGFβ that further stimulate Treg expansion and another is to stimulate adipocyte precursors to differentiate and stimulate wound contraction. As the inflammatory response is dampened and dead cells have been cleared, EpdSCs at the wound edge begin to migrate into the wound bed to re-epithelialize the epidermis. They are fueled by proliferating EpdSCs behind them to generate a one-two punch in repair.
