Figure 6. Therapeutic Applications of Skin and Muscle Stem Cells.
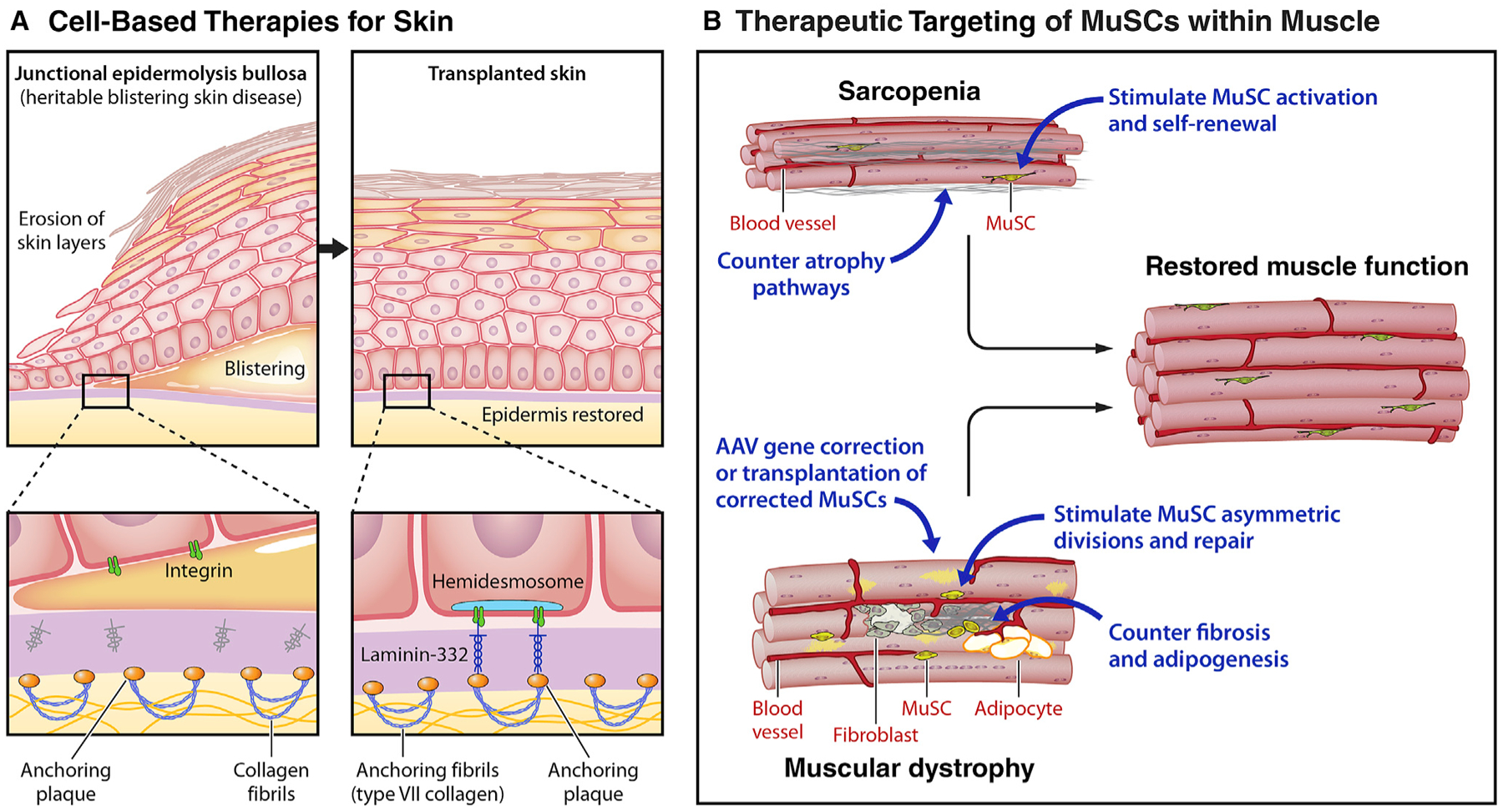
(A) Cell-based therapies for skin: epidermolysis bullosa. Recent advances in the treatment of the severe heritable blistering disorder, epidermolysis bullosa (EBD), highlight the potential for cell-based therapies for the skin. Epidermolysis bullosa is caused by mutations in components of the extracellular matrix, such as laminin 332 (LAMB3), that are essential for maintaining the adhesion of the epidermis to the dermis via type VII collagens. The loss of this adhesion results in severe blistering and skin compromise. By transplanting skin grafts produced from autologous epidermal stem cells obtained from patient biopsies and genetically corrected by transduction with retrovirus carrying functional LAMB3, 80% of the skin of an EB patient was replaced.
(B) Therapeutic targeting of MuSCs within muscle: sarcopenia and DMD. Due to the integral role MuSCs play in maintaining muscle function, therapies targeting MuSCs hold significant regenerative potential. (Top) Muscle strength commonly decreases with aging, a condition known as sarcopenia. To counter sarcopenia, potential therapeutic approaches entail targeting signaling pathways that are dysregulated in aged MuSCs to restore the regenerative function lost with aging. Alternative or combinatorial approaches seek to counter atrophy pathways in myofibers. (Bottom) Muscular dystrophies are heritable muscle wasting disorders that culminate in premature patient death due to the absence of crucial structural or contractile proteins. For Duchenne muscular dystrophy, a number of strategies entail restoring dystrophin by read through drugs that overcome premature stop codons, gene correction of the myofiber by adeno-associated virus (AAV)-mediated micro-dystrophin delivery, or CRISPR-Cas9-mediated exon skipping. These approaches could be used in combination with approaches to enhance MuSC function, e.g., stimulating asymmetric divisions or stem cell expansion, to enhance the rate of repair and ameliorate the disease. Anti-fibrotic agents that target FAPs could promote MuSC function by creating a favorable, pro-regenerative microenvironment.
