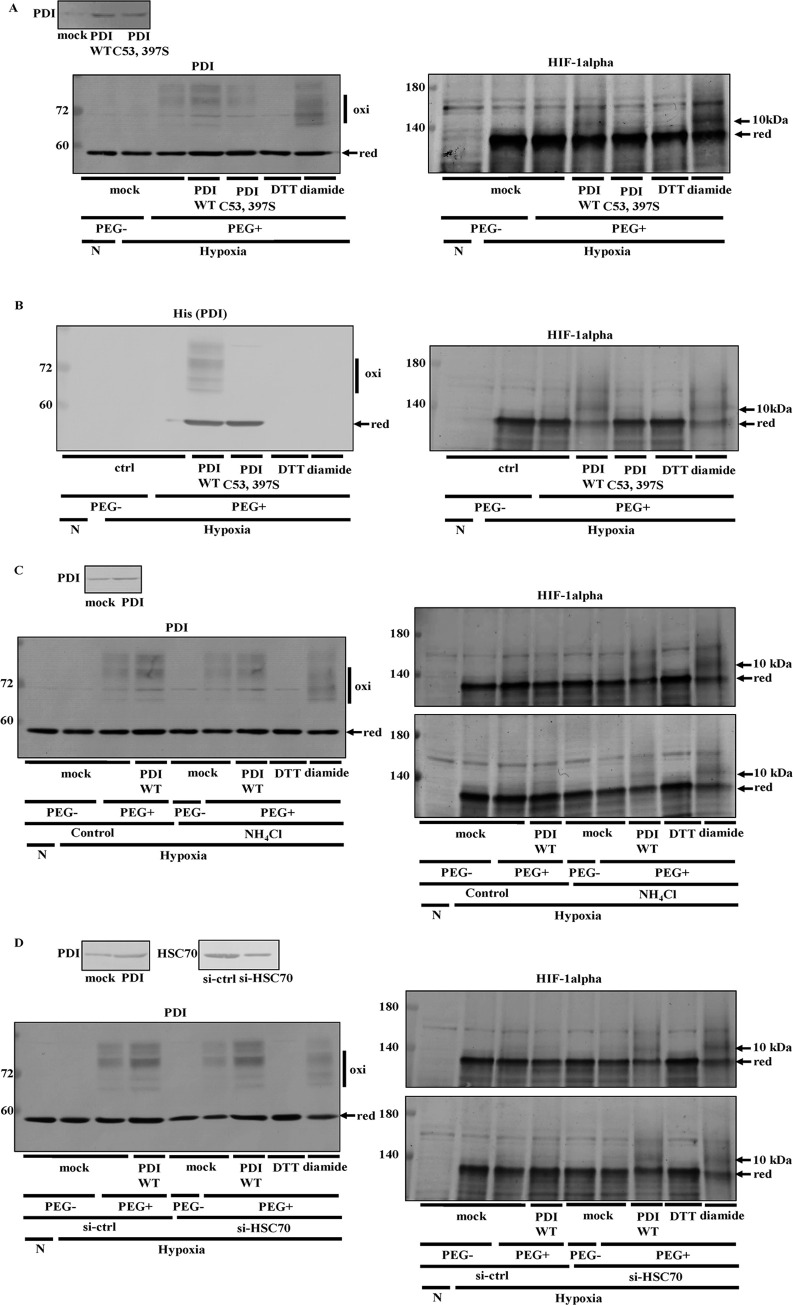
Fig 8. Detection of the HIF-1alpha redox state by PEG-maleimide.
(A) Hep3B cells overexpressing PDI or PDI C53, 397S were cultured under hypoxic conditions for 8 h in the presence of NH4Cl. (B) Hep3B cells were cultured under hypoxic conditions for 6 h and harvested with 50 mM Hepes, pH 7.5, containing 0.5% Nonidet P-40. The lysate from Hep3B cells was then treated with 17.5 nM purified His-tagged PDI WT or PDI C53, 397S. The redox states of proteins were detected using the same method as in Fig 4A. An up-shift in molecular weight by the binding of PEG-maleimide was detected by immunoblotting with the anti-PDI (A), -HIF-1alpha (A and B), or -His tag antibody (B). (C and D) PDI-overexpressing Hep3B cells were cultured for 8 h under hypoxic conditions in the presence of NH4Cl (C). PDI/pcDNA 3.1 (+) and si-HSC70 were transfected into Hep3B cells, and cells were cultured for 6 h under hypoxic conditions (D). Cells were harvested with PBS. The redox states of proteins were detected using the same method as that in Fig 4A. Since PDI decreased HIF-1alpha expression levels in the absence of NH4Cl (C) or in si-ctrl cells (D), 1.5-fold amount of protein was applied in Lane 4 in order to achieve an equal intensity in the reduced form of HIF-1alpha in other lanes. The knockdown of HSC70 was checked by immunoblotting with the anti-HSC70 antibody (upper panel).