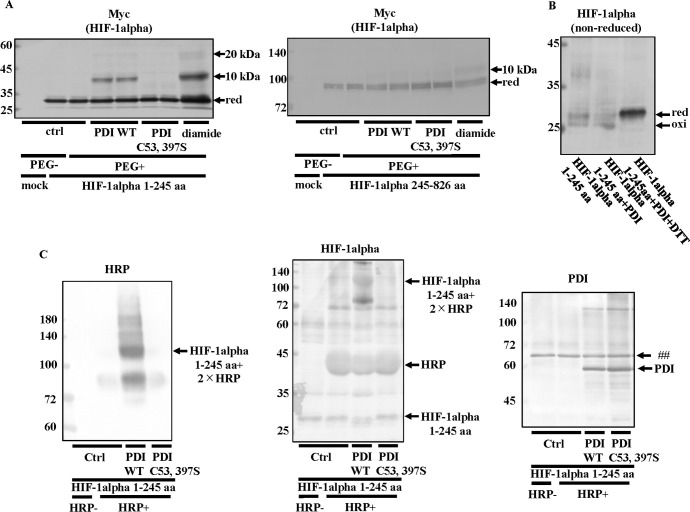
Fig 10. Detection of the HIF-1alpha (1–245 aa) redox state.
(A) Hep3B cells overexpressing HIF-1alpha 1–245 aa or HIF-1alpha 245–826 aa were cultured under hypoxic conditions for 6 h, and 17.5 nM purified His-tagged PDI WT or PDI C53, 397S was added to the lysate from Hep3B cells. The redox states of proteins were detected using the same method as in Fig 4A. An up-shift in molecular weight by the binding of PEG-maleimide was detected by immunoblotting with the anti-Myc antibody. (B) 350 nM purified His-tagged PDI was added to 350 nM His-tagged HIF-1alpha 1–245 aa, and proteins were incubated in the presence or absence of 10 mM DTT. Precipitated proteins were incubated with 50 mM NEM, and immunoblotting was performed with the anti-HIF-1alpha antibody. (C) 350 nM purified His-tagged PDI WT or PDI C53, 397S was added to 350 nM His-tagged HIF-1alpha 1–245 aa, and proteins were incubated with 20 mM NEM. After the removal of NEM, proteins were incubated with 1 mM DTT. Precipitated proteins were incubated with HRP Maleimide Conjugate. An up-shift in molecular weight by the binding of HRP was detected by immunoblotting with or without the anti-HIF-1alpha antibody. ##, non-specific band.