Abstract
The nematode C. elegans is a leading model to investigate the mechanisms of stress-induced behavioral changes coupled with biochemical mechanisms. Our group has previously characterized C. elegans behavior using a microfluidic-based electrotaxis device, and showed that worms display directional motion in the presence of a mild electric field. In this study, we describe the effects of various forms of genetic and environmental stress on the electrotactic movement of animals. Using exposure to chemicals, such as paraquat and tunicamycin, as well as mitochondrial and endoplasmic reticulum (ER) unfolded protein response (UPR) mutants, we demonstrate that chronic stress causes abnormal movement. Additionally, we report that pqe-1 (human RNA exonuclease 1 homolog) is necessary for the maintenance of multiple stress response signaling and electrotaxis behavior of animals. Further, exposure of C. elegans to several environmental stress-inducing conditions revealed that while chronic heat and dietary restriction caused electrotaxis speed deficits due to prolonged stress, daily exercise had a beneficial effect on the animals, likely due to improved muscle health and transient activation of UPR. Overall, these data demonstrate that the electrotaxis behavior of worms is susceptible to cytosolic, mitochondrial, and ER stress, and that multiple stress response pathways contribute to its preservation in the face of stressful stimuli.
Subject terms: Behavioural methods, Biological models, Stress and resilience, Behavioural genetics
Introduction
Organisms have evolved intricate molecular machineries in order to respond to adverse or stressful stimuli as their survival depends on their ability to continuously monitor the environment and mount appropriate responses. Since the initial work of Hans Selye in 1936, who studied stress response in rats and termed it ‘general adaptation syndrome’1,2, the work in the field of stress biology has grown exponentially, leading to a better understanding of the connections between stress and diseases. Animals exposed to stressful conditions such as heat, toxins, starvation, and psychological stress activate signaling cascades leading to physiological changes. For instance, in the larvae of fruit fly Drosophila melanogaster, noxious stimuli induce a stereotyped escape response mediated by neuronal signaling that manifests as rolling and bending reactions3. Work in rats showed that when animals were separated from their mothers as pups they showed increased stress response to environmental conditions4. Studies in humans have shown that stress can induce intracellular signaling leading to changes in prefrontal cortex function, thereby impacting learning and memory5. In addition, there are numerous reports on the endocrine system responding to stress via signaling cascades mediated by hormones such as adrenaline and cortisol6.
Detrimental stress in eukaryotes can often lead to an accumulation of misfolded proteins. The stress caused by this accumulation is mitigated by the molecular machinery consisting of several proteins that constitute the UPR signaling network7. The three well-known UPR pathways function in the cytosol, mitochondria, and ER, respectively7. The cytosolic heat shock response (HSR), UPRER, and UPRMT regulate both the expression and function of multiple chaperons to maintain homeostasis. These chaperons are members of the heat shock protein family and are transcriptionally regulated by the heat shock factor (HSF), which is a major player in the HSR. Together, UPRMT, UPRER, and HSR allay cellular stress by attenuating translation to maintain protein homeostasis within the cell, lowering reactive oxygen species (ROS), and inducing cell death7.
UPRMT is activated following mitochondrial dysfunction induced by misfolded protein accumulation within the mitochondria, and disrupts oxidative phosphorylation8. The response is similar across eukaryotes, although the regulation in mammals appears to be more complex8. Experiments in the nematode Caenorhabditis elegans have revealed that ATFS-1 (activating transcription factor associated with stress-1), an ATF5 homolog and bZip transcription factor family member, acts as a sensor protein9. ATFS-1 is normally degraded inside healthy mitochondria, but upon mitochondrial dysfunction is transported to the nucleus to regulate gene expression8. Failure to reduce mitochondrial stress can result in dysfunctional mitochondria which, if not cleared by the protective mechanism of mitophagy8,10, can lead to several deleterious conditions, including premature aging, sarcopenia, and cardiovascular disease10.
Similar to mitochondrial stress, ER stress is triggered by the accumulation of misfolded proteins in the ER. The UPRER response is facilitated by three different transmembrane proteins: inositol-requiring enzyme 1 (IRE1), protein kinase RNA (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which then activate distinct signaling pathways. There are significant redundancies between the pathways, with overlapping transcriptional activity. All three pathways regulate the expression of chaperones, including GRP78/BiP11. A key transcription factor that modulates UPRER signaling is the X-Box binding protein-1 (XBP1). While IRE1 is the main regulator of XBP1 activity, ATF6 and PERK also participate in IRE1-XBP1 signaling11,12. Failure to regulate ER stress can lead to diseases such as neurodegeneration, metabolic disorders, and cancer13,14.
Much of our knowledge of how stress affects behavior and the underlying signaling mechanisms have emerged from studies using a small set of model organisms, including C. elegans. This model nematode (worm) is a particularly attractive system due to its genetic tractability, ease of culture, and short life cycle, all of which serve to greatly expedite the rate of discovery15. Despite its relative simplicity, approximately half of C. elegans genes have homologs in humans and utilize many of the same processes16. Cellular stress can affect diverse processes in worms. Manipulations that increase mitochondrial and ER stress affect lifespan, fertility, susceptibility to diseases, and many behavioral processes17–19. The small size of the worm greatly facilitates experimentation using microfluidic devices20. Our lab has been investigating electrotaxis in C. elegans and how it is impacted by stress caused by genetic and environmental factors. In an earlier study, we demonstrated that a mild DC electric field in a microfluidic channel stimulates C. elegans to move towards the cathode in a directed manner, a response that is robust, highly repeatable, sensitive, and instantaneous21. Electrotactic movement serves as a powerful noninvasive response to evaluate the functional output of the nematode’s locomotory circuit following manipulation22. To understand whether the response is affected by treatments that increase stress, we exposed animals to different environmental conditions as well as investigated the impact of genetic mutations. Our findings show that chronic stress compromises electrotaxis speed whereas some forms of transient stress augments it. We found that worms exposed to chemicals that induce ER and mitochondrial stress exhibit reduced speed. A similar phenotype was also observed in mutant strains with disrupted mitochondrial and ER function. The essential role of HSR and UPRMT is further supported by our data showing that animals having defects in both atfs-1 and hsf-1 function have a significantly slower movement response. Our analysis of the UPRER genes reveals that ire-1/xbp-1 and pek-1 pathways play essential roles in regulating the electrotaxis of C. elegans. We also report that a polyglutamine enhancer-1 protein, PQE-1, maybe involved in the maintenance of HSR and mitochondrial and ER stress, likely due to its role in regulating the global protein synthesis23,24. To examine the sensitivity of electrotaxis speed to stress-inducing conditions, we exposed worms to chemical stressors, heat, different bacterial diets, and dietary restriction as well as daily exercise. The results showed that while chronic heat and reduced diet caused a decreased electrotactic movement, exercise had an increased response. Overall, our findings demonstrate that the electrotactic response of C. elegans is sensitive to cytosolic, mitochondrial, and ER stress. The results form the basis of future investigations of HSR, UPRMT, and UPRER signaling utilizing microfluidic electrotaxis as a functional output of nematode locomotor circuits.
Results
Paraquat-induced oxidative stress reduces the electrotaxis speed of C. elegans
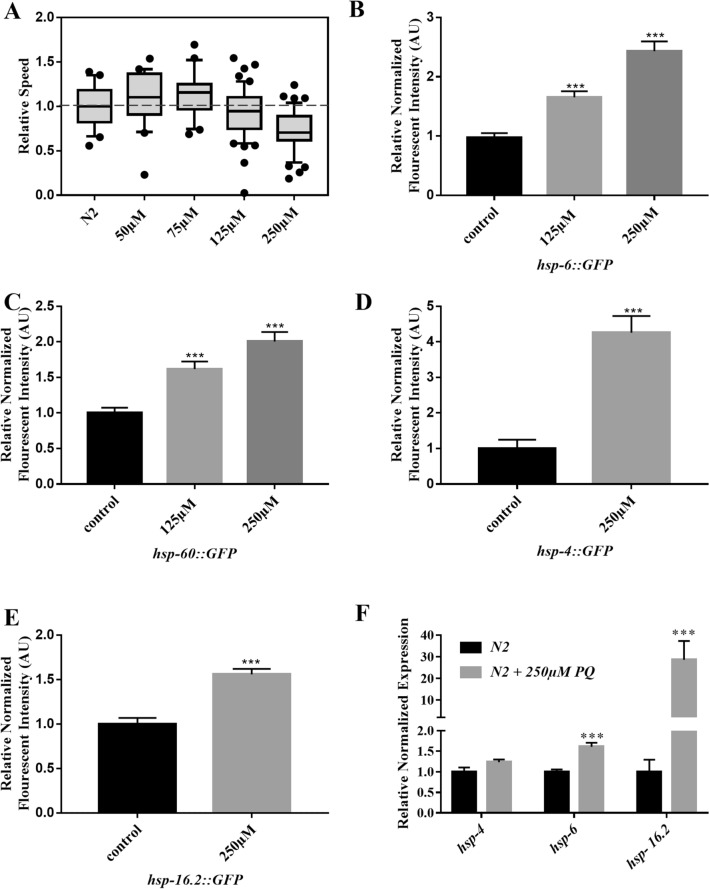
Oxidative stress is known to affect the behavioral responses of C. elegans17,18,25. To investigate whether this form of stress can also alter electrotaxis in animals, we used paraquat (PQ), a herbicide that disrupts mitochondrial function by inducing the generation of superoxides26,27. High concentrations of PQ have a detrimental effect on the lifespan of worms and cause damage to dopaminergic (DA) and other neurons by increasing ROS levels17,18,26–28. We examined the effect of PQ-induced stress on electrotaxis by exposing animals to different doses of the chemical. While exposures to low or moderate concentrations ranging from 50 to 125 µM had no effect on the movement of day-1 adults, 250 µM of PQ caused a significant defect, resulting in a slower speed in the microfluidic channel (Fig. 1A). In addition to electrotaxis, PQ-exposed animals showed increased expression of mitochondrial chaperones. Specifically, the mitochondrial stress response reporters hsp-6::GFP (HSP70 family) and hsp-60::GFP (HSP60 family) were significantly upregulated (Fig. 1B,C).
Figure 1.
Effect of PQ treatments on electrotaxis and stress response markers. Boxes represent measurements from 25 to 75th percentiles, central horizontal lines represent medians, vertical lines extend to 10th and 90th percentiles, and dots represent outliers. (A) Wild-type (N2) animals treated with PQ. PQ did not induce speed abnormalities at 50 μM (p = 0.128), 75 μM (p = 0.102), or 125 μM (p = 0.102), but resulted in speed deficits at 250 μM (p < 0.001). (B, C) Fluorescence intensity in animals expressing GFP under mitochondrial hsp-6 and hsp-60 promoters following PQ treatments. GFP fluorescence corresponding to both hsp-6::GFP and hsp-60::GFP reporters showed increased fluorescence following treatments with 125 μM (p < 0.001 in both cases) and 250 μM (p < 0.001 and p = 0.003 respectively) PQ. (D, E) Fluorescence intensity of PQ-treated hsp-4::GFP and hsp-16.2::GFP animals. Fluorescence was increased following treatment with 250 μM PQ (p < 0.001 in both cases). (F) Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) analysis showed an increase in hsp-6 (p < 0.0001) and hsp-16.2 (p = 0.0007) but not hsp-4 (p = 0.1125) transcripts in N2 day-1 adults following exposure to 250 μM PQ. Number of animals were (A) N2 untreated: n = 80, N2 + 50 μM PQ: n = 20, N2 + 75 μM PQ: n = 20, N2 + 125 μM PQ: n = 50, N2 + 250 μM PQ: n = 45. (B) hsp-6::GFP untreated: n = 40, hsp-6::GFP + 125 μM PQ: n = 32, hsp-6::GFP + 250 μM PQ: n = 31, (C) hsp-60::GFP untreated: n = 33, hsp-60::GFP + 125 μM PQ: n = 34, hsp-60::GFP + 250 μM PQ: n = 35. (D) hsp-4::GFP untreated: n = 27, hsp-4::GFP + 250 μM PQ: n = 29, (E) hsp-16.2::GFP untreated: n = 31, hsp-16.2::GFP + 250 μM PQ: n = 20. (F) N2 untreated: n = 2 batches, N2 + 250 μM PQ: n = 2 batches. Statistical analyses for panels A-E were done using one-way ANOVA with Dunnett’s post hoc test. qPCR results in F were generated by BioRad’s CFX Maestro software (version 3.1, https://www.bio-rad.com/en-ca/category/qpcr-analysis-software) and data was analyzed using one-way ANOVA with Tukey’s post hoc test. AU: Arbitrary Unit.
To determine whether PQ affects other processes, we examined the expression of two other chaperones for ER and cytosolic stress, namely HSP-4 (BiP/GRP78 family member, ER stress) and HSP-16.2 (HSP16 family, cytosolic stress) following PQ treatment. Although the GFP reporters for both these chaperons showed a statistically significant increase in fluorescence, the fold change of the ER GFP reporter was much higher than that of mitochondrial reporters (Fig. 1D,E). Similar to changes in fluorescence reporters, the transcript analysis also showed higher levels of hsp-6 and hsp-16.2 following PQ exposure (Fig. 1F). We did not see a significant increase in hsp-4 transcript. These results are consistent with the previously reported finding that PQ induces both mitochondrial and ER stress in different animal models29–31. Overall, PQ causes multiple stresses in C. elegans that contribute to a decrease in electrotaxis speed.
Mutations in genes regulating mitochondrial function affect electrotaxis
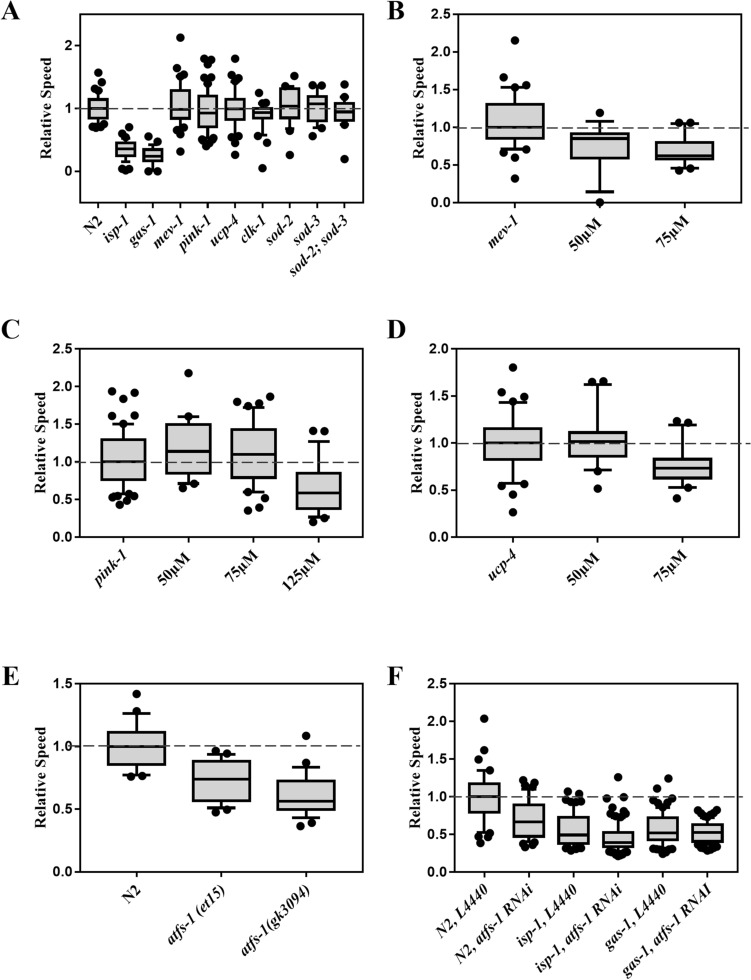
To further investigate whether the electrotactic response is perturbed by mitochondrial dysfunction, we conducted a survey of mutants affecting mitochondrial processes and UPRMT. The set of genes consisted of an ortholog of ubiquinol-cytochrome C reductase (isp-1, Rieske iron sulfur protein subunit of mitochondrial complex III), a subunit of mitochondrial complex I (gas-1), a subunit of mitochondrial complex II (mev-1, cytochrome b), PTEN-induced kinase 1 (pink-1), mitochondrial uncoupling protein (UCP) ortholog (ucp-4), two mitochondrial superoxide dismutase orthologs (sod-2 and sod-3), coenzyme Q7 ortholog (clk-1), and atfs-1. Some of these genes are reported to affect motility of C. elegans on solid media and thrashing in liquid environment32–35.
Analysis of the electrotactic response showed that most of the mutant animals, including mev-1(kn1), pink-1(ok3538), ucp-4(ok195), clk-1(e2519), sod-2(gk257), sod-3(tm760), and sod-2(gk257); sod-3(tm760) double, had no obvious defects; however, two particular mutants, isp-1(qm150) and gas-1(fc21), showed a significantly reduced electrotaxis speed (Fig. 2A). Although both genes encode components of the electron transport chain, isp-1(qm150) is long-lived with resistance to oxidative damage36, while gas-1(fc21) is short-lived with increased ROS and sensitivity to oxidative damage37, suggesting that neither lifespan differences nor ROS toxicity correlate with electrotaxis phenotypes. This notion is supported by the other mutant data, indicating that mev-1(kn1) has increased ROS and oxidative damage with short lifespan, clk-1(e2519) shows decreased oxidative damage and long lifespan, and sod mutants show increased oxidative damage with normal or extended lifespans, yet all exhibit similar electrotaxis speeds (Fig. 2A)38,39.
Figure 2.
Electrotaxis of mitochondrial mutants. Refer to Fig. 1 for a description of box plot. (A) isp-1(qm150) (p < 0.001) and gas-1(fc21) (p < 0.001) animals exhibit speed defects. In contrast, mev-1(kn1) (p = 0.567), pink-1(ok3538) (p = 0.239), ucp-4(ok195) (p = 0.942), clk-1(e2519) (p = 0.065), sod-2(gk257) (p = 0.509), sod-3(tm760) (p = 0.578), and sod-2(gk257); sod-3(tm760) double (p = 0.485) mutants are comparable to the wild-type (N2) control. (B) mev-1(kn1) animals exhibited significant slowness following treatment with 50 μM (p = 0.003) or 75 μM (p < 0.001) PQ. (C) pink-1(ok3538) animals showed no obvious defect following treatment with 50 μM (p = 0.394) or 75 μM (p = 0.694) PQ, but were significantly slower following treatment with 125 μM PQ (p < 0.001). (D) ucp-4(ok195) animals were normal following treatment with 50 μM (p = 0.946), but showed significant slowness when treated with 75 μM PQ (p = 0.002). (E) atfs-1(et15) and atfs-1(gk3094) animals showed defective electrotaxis phenotype (p < 0.001 and p = 0.008, respectively). (F) atfs-1 RNAi resulted in a significant decrease in the electrotaxis speed of N2 (p < 0.001) and isp-1(qm150) (p = 0.0480) animals whereas it had no effect on the speed of gas-1(fc21) (p = 0.7929) animals. The numbers of animals were (A) N2: n = 40, isp-1(qm150): n = 39, gas-1(fc21): n = 29, mev-1(kn1): n = 40, pink-1(ok3538): n = 61, ucp-4(ok195): n = 40, clk-1(e2519): n = 30, sod-2(gk257): n = 21, sod-3(tm760): n = 20, sod-2(gk257); sod-3(tm760): n = 20. (B) N2: n = 29, atfs-1(et15): n = 26, atfs-1(gk3094): n = 26. (C) mev-1(kn1) untreated: n = 20, + 50 μM PQ: n = 18, + 75 μM PQ: n = 20. (D) pink-1(ok3538) untreated: n = 33, + 50 μM PQ: n = 20, + 75 μM PQ: n = 40, + 125 μM PQ: n = 27. (E) ucp-4(ok195) untreated: n = 20, + 50 μM PQ: n = 20, + 75 μM PQ: n = 20. (F) N2, L4440: n = 45, N2, atfs-1 RNAi: n = 44, isp-1(qm150), L4440: n = 60, isp-1(qm150), atfs-1 RNAi: n = 93, gas-1(fc21), L4440: n = 76, gas-1(fc21) atfs-1 RNAi: n = 71. In all cases statistical analyses were done using one-way ANOVA with Dunnett’s post hoc test.
We chose a subset of mitochondrial genes for further investigation. Specifically, we explored the possibility that mutant animals may not exhibit an electrotaxis phenotype on their own, but may do so when subjected to further stress. Consistent with this, mev-1(kn1) and pink-1(ok3538), which show sensitivity to PQ18,38, displayed electrotaxis defects at exposure concentrations that do not alter the speed of control animals. Specifically, mev-1(kn1) animals exhibited speed deficits when cultured under chronic exposures to either 50 μM or 75 μM PQ (Fig. 2B). Similarly, pink-1(ok3538) animals were also affected, displaying significantly reduced electrotaxis speed at 125 μM PQ (Fig. 2C). Interestingly, ucp-4(ok195) adults displayed increased sensitivity to PQ under our chronic exposure paradigm, exhibiting slow electrotaxis at 75 μM (Fig. 2D). Thus, PQ-induced stress and mitochondrial defects perturb C. elegans electrotaxis speed.
To further investigate the effect of perturbations in UPRMT on electrotaxis, we examined the phenotypes of two different atfs-1 alleles, a genetic null atfs-1(gk3094) and a gain-of-function atfs-1(et15). While the gk3094 mutation prevents the UPRMT response, an opposite phenotype is caused by et1540–42. Additionally, the HSR is activated in gk3094 animals41. Both atfs-1 mutants displayed significantly slower speeds than wild-type controls, although the null allele phenotype was most severe (Fig. 2E). We also knocked down atfs-1 in isp-1 and gas-1 mutant strains and found that while the electrotaxis speed of isp-1(qm150) was reduced, there was no change in gas-1(fc21) animals (Fig. 2F). Collectively, these data demonstrate that electrotaxis speed is sensitive to disruptions in mitochondrial function, UPRMT, and HSR.
Impairment of UPRER causes electrotaxis speed deficits
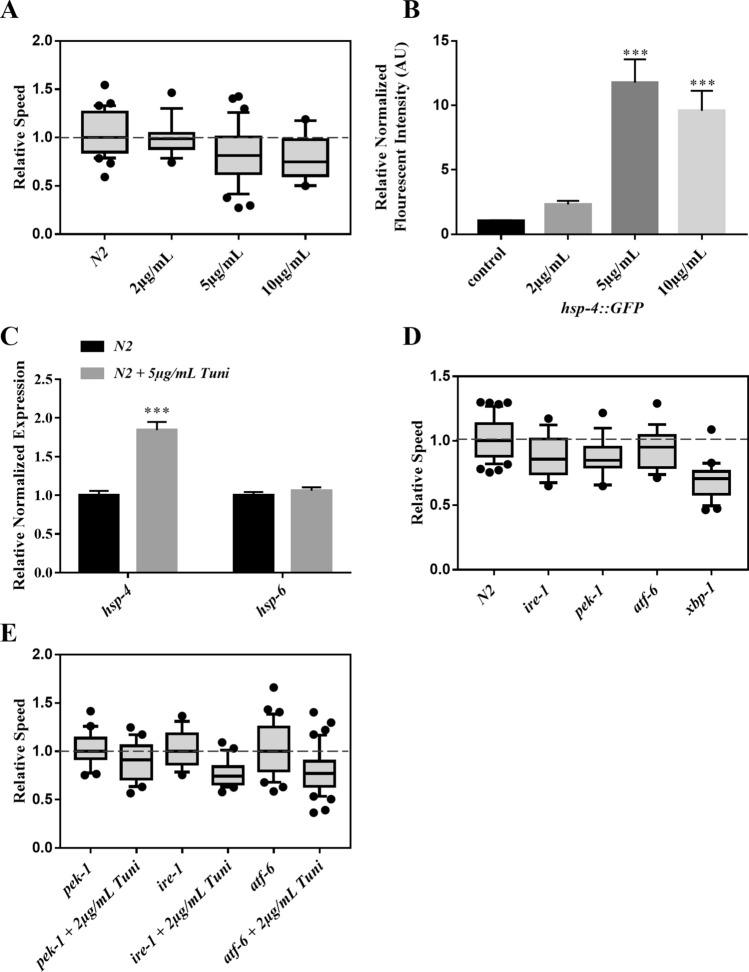
Our finding that PQ induces the expression of ER chaperone hsp-4::GFP (Fig. 1C) led us to investigate the impact of ER stress on electrotaxis using tunicamycin, a chemical that promotes protein misfolding by inhibiting protein glycosylation43,44. As shown in Fig. 3A, chronic exposure to tunicamycin at concentrations of 5 μg/mL or greater significantly reduced electrotaxis speed. These results are supported by the analysis of hsp-4::GFP reporter expression and hsp-4 transcript (Fig. 3B,C), suggesting the detrimental effect of tunicamycin exposure40. As expected, hsp-6 level was unchanged (Fig. 3C). We did not test electrotactic responses of animals at concentrations higher than 10 μg/mL due to significant toxicity, as judged by the highly pronounced larval lethality and slow growth of escapers at this concentration (data not shown).
Figure 3.
Electrotaxis phenotype of tunicamycin-treated animals and UPRER mutants. Refer to Fig. 1 for a description of box plot. (A) Wild-type (N2) animals show no speed abnormalities when exposed to 2 μg/mL of tunicamycin (p = 0.590), but exhibit speed deficits at higher doses, 5 μg/mL (p = 0.002) and 10 μg/mL (p = 0.008). (B) Fluorescence intensity of tunicamycin-treated hsp-4::GFP animals. The fluorescence was significantly increased following treatments with 5 μg/mL, and 10 μg/mL tunicamycin (p < 0.001). C) RT-qPCR analysis of chaperons hsp-4 (p = 0.003) and hsp-6 (p = 0.3652) in N2 day-1 adults following exposure to 5 μg/mL of Tunicamycin. (D) Electrotaxis speed of N2 and UPRER mutants. While ire-1(v33) (p = 0.0144), pek-1(ok275) (p = 0.0082) and xbp-1(zc12) (p = 0.0001) animals exhibited significant speed defects, atf-6(ok551) mutants were not significantly affected (p = 0.2995). (E) Responses of mutants following 2 μg/mL tunicamycin treatments., Animals showed a significant decline in the speed. P values are 0.0333 for pek-1(ok275), < 0.0001 for ire-1(v33), and 0.0004 for atf-6(ok551). The numbers of animals were: (A) N2 untreated: n = 30, N2 + 2 μg/mL Tuni: n = 15, N2 + 5 μg/mL Tuni: n = 32, N2 + 10 μg/mL Tuni: n = 10. (B) hsp-4::GFP untreated: n = 45, hsp-4::GFP + 2 μg/mL Tuni : n = 32, hsp-4::GFP + 5 μg/mL Tuni: n = 37, hsp-4::GFP + 10 μg/mL Tuni: n = 31. (C) N2 untreated: n = 3 batches, N2 + 5 μg/mL Tuni: n = 3 batches. (D) N2: n = 42, ire-1: n = 17, pek-1: n = 18, atf-6: n = 18, xbp-1: n = 23. (E) pek-1: n = 18, pek-1 + 2 μg/mL Tuni: n = 20, ire-1: n = 17, ire-1 + 2 μg/mL Tuni: n = 21, atf-6: n = 33, atf-6 + 2 μg/mL Tuni: n = 44. One-way ANOVA was performed followed by Dunnett’s post hoc test for A and B, which showed that all conditions except one (i.e., control vs. 2 µg in panel B, p = 0.7410) were significant. Student’s unpaired t-test was used for the remaining drug conditions. Data in C was analyzed using one-way ANOVA with Tukey’s post hoc test.
Our group had previously demonstrated the role of dopaminergic neurons in mediating electrotaxis22. Therefore, we examined whether tunicamycin-induced ER stress will affect these neurons in our assay. The analysis of dat-1::YFP reporter revealed visible defects in neuronal morphology in treated animals illustrated by dendritic/axonal abnormalities and loss of cell bodies (Fig. S1A). A similar result was obtained following PQ treatment (Fig. S1B). These data suggest that toxin-induced neuronal damage may contribute to electrotaxis deficits in animals.
We further characterized ER disruption by testing the phenotypes of UPRER pathway mutants, specifically ire-1, pek-1, atf-6, and xbp-1. The speed was significantly lower in ire-1(v33), xbp-1(zc12), and pek-1(ok275) but not atf-6(ok551) animals compared to the control, (Fig. 3D). Interestingly, the defect was most pronounced in xbp-1 mutants, which may be due to the gene being regulated by multiple UPRER arms. We also examined the sensitivity of UPRER mutants by exposing them to tunicamycin. Treatment with 2 μg/mL caused a significant decrease in the mobility of all three strains (Fig. 3E). A higher concentration (5 μg/mL) was found to be toxic to worms, resulting in lethality (data not shown). Taken together, the results show that electrotaxis is susceptible to ER stress-inducing conditions, and all three UPRER transducers function to facilitate this behavior.
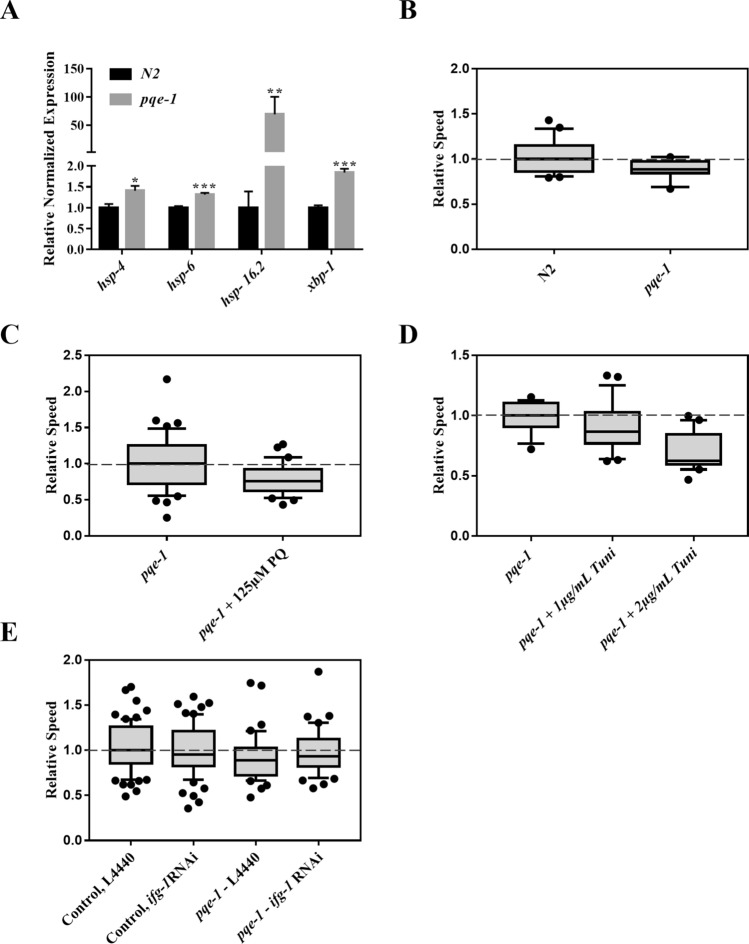
Next, the role of pqe-1 was investigated based on its potential link to ER stress. Earlier, pqe-1 mutants were reported to cause an increase in transgene expression and enhance polyglutamine (poly-Q) neurotoxicity23,24. We speculated that both these phenotypes may be caused by proteotoxic stress due to a failure to regulate global protein synthesis. In support of this, pqe-1(ok1983) animals showed an increase in the expression of hsp-4 chaperon and xbp-1 (Fig. 4A, Fig. S2A). Interestingly, hsp-6 and hsp16.2 levels were also upregulated (Fig. 4A). As expected, the electrotaxis speed of mutant animals was significantly reduced (Fig. 4B), and the phenotype was exacerbated by treatment with PQ and tunicamycin (Fig. 4C,D). The sensitivity towards tunicamycin was particularly pronounced; although wild-type animals showed no abnormal electrotaxis phenotype at 2 μg/mL tunicamycin, pqe-1 mutants exhibited a significantly reduced speed (compare Fig. 4D with 3A). Concentrations of tunicamycin above 2 μg/mL induced growth arrest and lethality in pqe-1(ok1983) worms (data not shown). The mutant animals also exhibited an increased degeneration of dopaminergic neurons and shorter lifespan (Fig. S2B,C).
Figure 4.
Analysis of pqe-1 mutants under different stress-inducing conditions. Refer to Fig. 1 for a description of box plot. (A) RT-qPCR analysis showing increased expression of hsp-4 (p = 0.0128), hsp-6 (p < 0.0001), hsp-16.2 (p = 0.0012) and xbp-1(p < 0.0001) in pqe-1(ok1983) day-1 adults. (B) pqe-1(ok1983) animals exhibited a reduced electrotaxis speed compared to wild-type N2 (p = 0.0053) (C) The speed was further decreased following treatments with 125 μM PQ (p = 0.007). (D) The speed of pqe-1(ok1983) was not significantly different from untreated control (p = 0.2461). At 2 μg/mL exposure, pqe-1 mutants showed a significant decrease in speed (p = 0.0001). (E) The electrotaxis speed of ifg-1 RNAi in control and pqe-1(ok1983) animals. The ifg-1 RNAi had no effect on control animals (p = 0.4193) or pqe-1 mutants (p = 0.6676). The numbers of animals were (B) N2: n = 3 batches, pqe-1 n = 3 batches. (C) pqe-1 untreated: n = 44, pqe-1 + PQ 125 μM: n = 30. (D) pqe-1: n = 16, pqe-1 + 1 μg/mL Tuni n = 21, pqe-1 + 2 μg/mL Tuni n = 21. (E) Control, L4440: n = 47, Control, ifg-1 RNAi: n = 66, pqe-1, L4440: n = 41, pqe-1, ifg-1 RNAi: n = 44. Data was analyzed using one-way ANOVA with Tukey’s post hoc test (A), one-way ANOVA with Dunnett’s post hoc test (D), and unpaired Student’s t-test (B,C,E).
We investigated whether reducing protein translation would lower proteotoxicity leading to a rescue of the electrotaxis defect of pqe-1 mutant animals. This was tested by knocking down the eIF4G homolog, ifg-1, that encodes a component of the eIF4 complex45. The results showed that ifg-1 RNAi was unable to suppress the phenotype of pqe-1 mutant (Figs. 4E, S3). Overall, these observations provide the first evidence that pqe-1 plays an essential role in maintaining multiple stress response pathways and electrotactic response of C. elegans.
Chronic heat impairs the electrotactic response
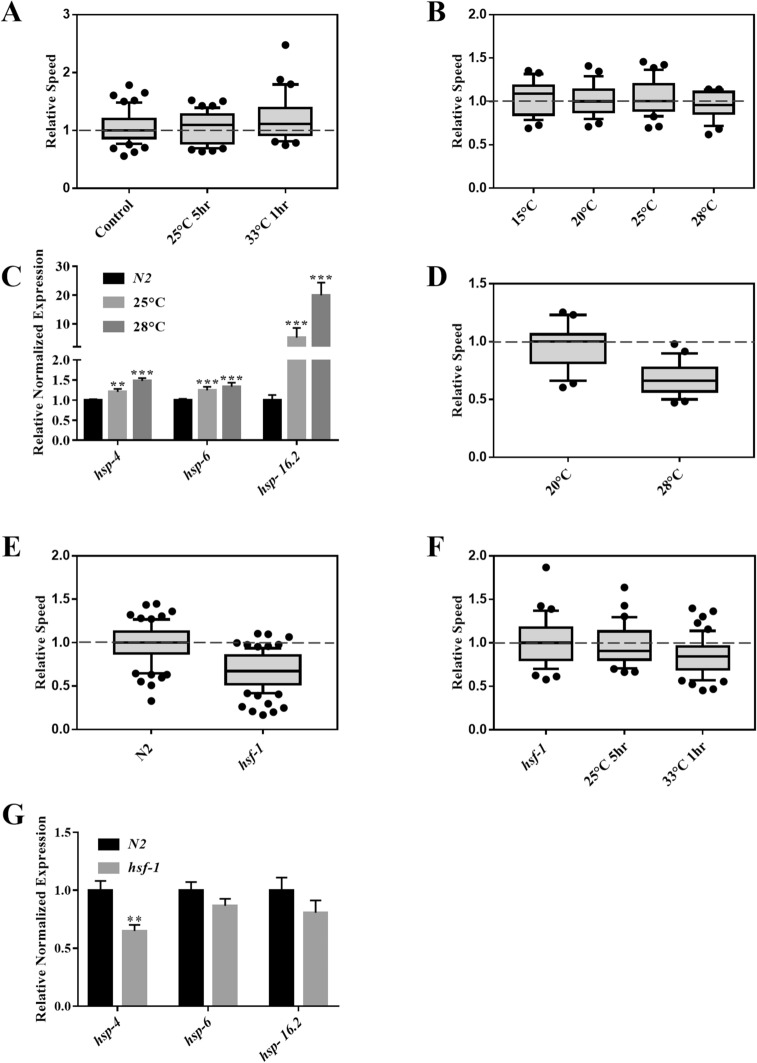
Studies have shown that heat treatments can have both positive and negative effects on animals due to activated HSR7,46 and UPRER7,47. We investigated the electrotactic movement of young adults that were subjected to acute and chronic heat conditions. Worms exposed to two types of heat pulses, namely 5 h at 25 °C and 1 h at 33 °C, showed no change in their speed (Fig. 5A). Three additional treatments with a longer exposure time (i.e., 8 h each at 15 °C, 25 °C, and 28 °C), did not affect the speed either (Fig. 5B); however, all three heat shock chaperons (hsp-4, hsp-6, and hsp-16.2) were significantly upregulated (Figs. 5C, S4), suggesting the activation of UPR pathways. By contrast, chronic heat treatment at 28 °C for 3 days significantly reduced the speed (Fig. 5D). This demonstrates that unlike shorter duration (up to 8 h) of heat pulses, prolonged heat stress affects the electrotaxis speed of C. elegans.
Figure 5.
Effect of temperature on electrotactic response. Refer to Fig. 1 for a description of box plot. (A) Phenotype of animals exposed to two different heat conditions. No significant difference in speed was observed for any of the treatments (25 °C for 5 h, p = 0.970; 33 °C for 1 h, p = 0.101). (B) Electrotaxis of wild-type day-1 adults following exposure to heat, each for an 8 h period. No significant change was observed in the speed at 15 °C (p = 0.9215), 25 °C (p = 0.9026) and 28 °C (p = 0.3925). (C) RT-qPCR analysis of hsp-4, hsp-6 and hsp-16.2 in wild-type day-1 adults following 8 h exposures to 25 °C and 28 °C. The expression of the UPR chaperones was significantly increased following both treatments (p < 0.001). (D) Electrotaxis speed of wild-type animals following a chronic exposure at 28 °C for 3 days. Speed was significantly decreased (p < 0.0001) compared to the control grown at 20 °C. (E) hsf-1(sy441) animals exhibited significant slowness (p < 0.001). (F) hsf-1(sy441) animals exposed to 33 °C for 1 h exhibit significant slowness (p = 0.0017) while those exposed to 25 °C for 5 h had no obvious change (p = 0.6532). . (G) RT-qPCR analysis of hsp-4, hsp-6 and hsp-16.2 in hsf-1 mutants. The expression of hsp-4 was reduced (p < 0.01) however hsp-6 (p = 0.145032) and hsp-16.2 (p = 0.234128) were unaffected. The numbers of animals were (A) Control, untreated: n = 57, 25 °C for 5 h: n = 49, 33 °C for 1 h: n = 31. (B) 20 °C control: n = 26, 15 °C: n = 29, 25 °C: n = 35, 28 °C: n = 24. (C) Control : n = 3 batches; 25 °C: n = 3 batches, and 28 °C: n = 3 batches. (D) 20 °C control and 28 °C: n = 23. (E) N2: n = 70, hsf-1(sy441): n = 90. (F) hsf-1(sy441) untreated: n = 63, hsf-1(sy441) 25 °C for 5 h: n = 51, hsf-1(sy441) 33 °C for 1 h: n = 31. (G) N2: n = 3 batches; hsf-1(sy441): n = 3 batches. Statistical analyses were performed using one-way ANOVA with Dunnett’s post hoc test (A,B,F), Student’s t-test (D,E), and one-way ANOVA with Tukey’s post hoc test (C,G).
One of the ways by which heat can induce stress is by interfering with the function of the heat shock transcription factor (HSF)7. In C. elegans, the HSF ortholog hsf-1 is transcriptionally activated by a variety of stress conditions, including heat exposure48. In support of hsf-1′s involvement, we found that hsf-1(sy441) mutants have a significant electrotaxis speed deficit (Fig. 5E), which was further enhanced by a 1 h heat pulse at 33 °C but not by 5 h at 25 °C (Fig. 5F). There was no change in hsp-6 and hsp-16.2 levels in hsf-1 mutant animals, although hsp-4 was significantly down-regulated (Fig. 5G). Given that hsf-1 regulates the expression of a large number of genes49, we speculate that other heat shock chaperon(s) might mediate hsf-1 function in electrotaxis. We also found that the hsf-1(sy441) phenotype was exacerbated by PQ-induced stress and L1 starvation (Figs. S5, S6), which indicates that hsf-1 is a key regulator of diverse stress-induced responses.
Electrotactic movement decreases with reduced diet but increases after exercise
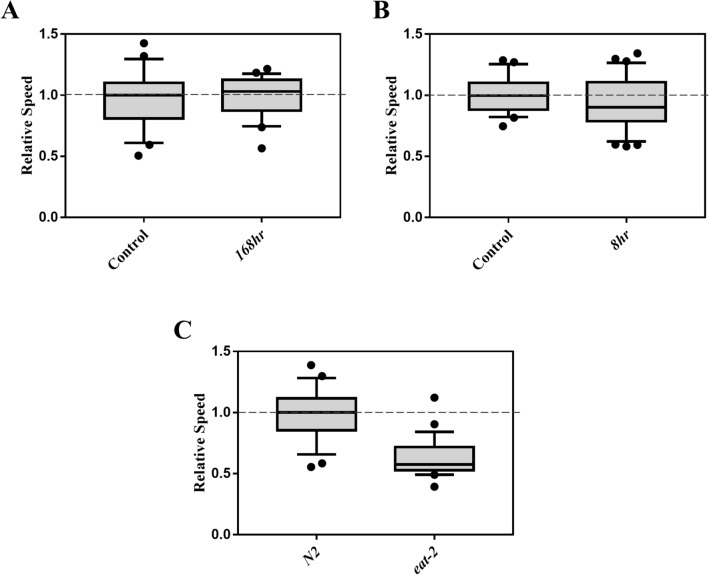
Another form of stress that we tested is starvation. Initially, the effects of two different starvation exposure protocols on electrotaxis were investigated. In one case, L1 larvae were subjected to starvation for 168 h and subsequently allowed to resume development in the presence of food. In the second case, day-1 adults were exposed to acute starvation conditions. Both nutrient deprivation treatments showed that the effect on electrotaxis was comparable to that of the controls (Fig. 6A,B).
Figure 6.
Effect of starvation on the electrotactic movement. Refer to Fig. 1 for a description of box plot. (A) Electrotaxis of animals starved for 168 h during the L1 larval stage. The speed was unaffected (p = 0.660). (B) Electrotaxis of day-1 adult wild-type animals starved for 8 h. The speed was comparable to the control (p = 0.3350). (C) The speed of eat-2(ad1116) adults was significantly reduced (p < 0.0001). The numbers of animals were (A) Control n = 21, 168 h n = 21. (B) Control n = 22, 8 h n = 25. (C) N2 day-1 adults n = 25, eat-2(ad1116) n = 29. Statistical Analysis was performed using unpaired Student’s t-test.
We also investigated a chronic form of nutrient deprivation that involves reduced daily caloric intake. This treatment, termed ‘dietary restriction (DR),’ has been shown to be beneficial in animals such as lifespan extension, increased metabolic fitness, and reduced severity of age-related physiological decline50–52. To this end eat-2 mutants were examined. eat-2(ad1116) animals have a longer lifespan that is attributed to chronic DR due to their slower pharyngeal pumping33. We found that the mutants had a significantly slower speed, suggesting that DR affects electrotaxis behavior (Fig. 6C). Since eat-2 mutants show higher fluorescence based on hsp-4::GFP reporter analysis53 but no change in hsp-6::GFP54, we conclude that the sensitivity of electrotactic response to dietary restriction is likely due to the detrimental effect of persistent ER stress.
In addition to starvation and DR, we explored the effect of dietary changes on electrotaxis. Two of the bacterial strains that were initially tested were Streptomyces venezuelae and Bacillus thuringiensis. The metabolites secreted by these bacteria cause intracellular stress in C. elegans55,56. We found that neither bacteria had an impact on electrotaxis (Fig. S7). Next, worms were fed with two plant pathogens, Agrobacterium tumefaciens and Pectobacterium carotovorum57–59 and a nonpathogenic fungus, Cryptococcus aquaticus. Plant pathogens have been reported to have a detrimental effect on the lifespan of worms60. None of these cultures had an obvious effect on electrotactic movement (Fig. S8). We also tested three commonly used E. coli strains, HB101, HT115, and DH5α, but, once again, observed no change in movement (Fig. S8). These results led us to conclude that electrotaxis behavior is unaffected by the above selected set of microorganisms, although the possibility of reduced nutrient intake in some or all cases cannot be ruled out. In the future, a larger set of microbial diets should be tested to investigate their effect on animals more thoroughly.
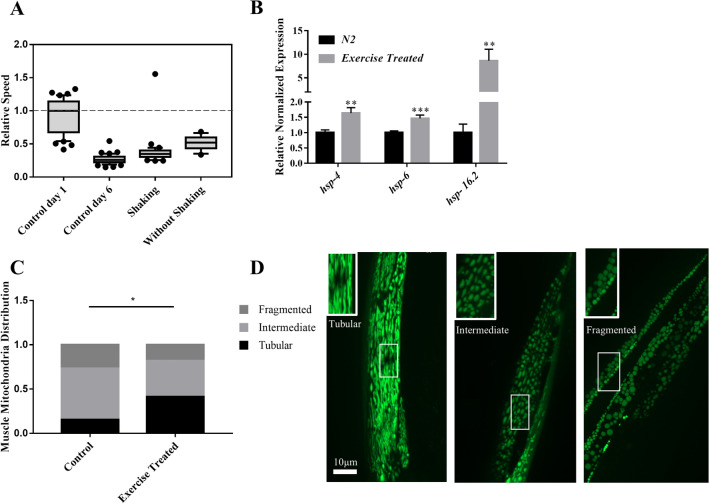
Finally, we investigated the effect of physical activity on the electrotactic movement of animals. The beneficial effects of exercise in humans and model organisms are well documented. In addition to improving muscle fitness, exercise also promotes neuronal health61–63. Since swimming activity in C. elegans induces known features of exercise reported in mammalian systems62,64,65, worms were allowed to swim in M9 for 30 min daily, starting at the L3 larval stage, for a period of 7 days (see Methods for details). The results showed that animals had a significantly faster electrotaxis speed compared to controls of the same age, although they were slower compared to day-1 controls (Fig. 7A). Consistent with this, all three heat shock chaperons, hsp-4, hsp-6, and hsp-16.2, were upregulated (Fig. 7B). Additionally, we found that exercise caused a significant increase in the proportion of animals with tubular muscle mitochondria62,66 (Fig. 7C,D). There was no change in dopaminergic neurons (Fig. S9).
Figure 7.
Effect of Exercise on electrotaxis reponse. (A) Worms were subjected to swimming exercise. A significant increase in the speed was observed using two different treatments (with shaking and without shaking: p < 0.001) compared to same age control. The speed was slower than day-1 controls (p < 0.0001). (B) RT-qPCR analysis of hsp-4, hsp-6 and hsp-16.2 in wild-type adults following 7 days of shaking exercise treatment. The expression of all three chaperones was significantly increased (p < 0.01). (C, D) Muscle mitochondria morphology in day-6 myo-3::GFP(mit) adults after shaking exercise. Animals were placed into three different categories, i.e., having mostly tubular, fragmented, and intermediate (i.e., combination of tubular and fragmented) shapes of mitochondria. These shapes correspond to mitochondrial network being normal (tubular) and defective (fragmented and intermediate). Exercise resulted in significantly more animals having tubular mitochondria (p = 0.045). The numbers of animals were: (A) Control day-1: n = 41, Control day-6: n = 48, shaking: n = 36 , without shaking: n = 14. (B) N2: n = 3 batches, exercise treated: n = 3 batches, (C) Control: n = 23, exercise treated: n = 23. Data was analyzed using one-way ANOVA with Dunnett’s post hoc test (A), one-way ANOVA with Tukey’s post-hoc test (B), and Chi-square test (C).
Discussion
The experiments described herein are the first to demonstrate that the electrotactic response of C. elegans is affected by mutations and environmental conditions that increase cytosolic, mitochondrial, and ER stress. We found that the electrotaxis speed was altered by treatment with PQ and tunicamycin, two chemicals that are known to negatively impact the locomotory behavior of worms on solid media17,67. PQ exposure causes generation of ROS, which activates the expression of multiple chaperones such as hsp-4 (ER stress), hsp-6 and hsp-60 (both mitochondrial stress), and hsp-16.2 (cytosolic stress). The effects of PQ on mitochondrial and ER stress are well documented; hence, the effects of PQ on electrotaxis are likely due to increased stress associated with these two organelles13,30,68.
Independent of ROS, our data show that mutations that cause mitochondrial stress also lead to decreased electrotaxis speed. The slow speed of atfs-1(gf) mutants indicates that the UPRMT contributes to maintaining the wild-type behavior. Analysis of ucp-4, mev-1, and pink-1 mutants revealed that exposure to a relatively low concentration of PQ induced speed defects. In mammalian cells, mutations in UCP genes are associated with increased ROS and oxidative stress69–71. Interestingly, Iser and colleagues (2005) reported earlier that ucp-4(ok195) worms exhibit wild-type lifespan and survival in the presence of PQ, although the animals display an elevated ATP level36,72. Our finding that ucp-4 mutants have defects in electrotaxis suggests that the movement response of animals may be sensitive to ROS levels. Future studies directly measuring oxidative damage following PQ exposure in different genetic backgrounds will be helpful in illuminating the relationship between ROS and electrotaxis.
isp-1 and gas-1 mutants exhibit reduced electrotaxis speed. While mutations in both these genes are also associated with electron transport hindrance and increased ROS generation, the isp-1 mutant actually exhibits oxidative stress resistance due to low oxygen consumption and increased expression of mitochondrial superoxide dismutase SOD-336. On the other hand, gas-1 mutants are hypersensitive to oxidative stresses such as PQ36,37. The lifespan phenotypes of isp-1 and gas-1 mutants are also opposite, wherein isp-1 animals have a long lifespan and gas-1 are short-lived, possibly due to differences in ROS production and ROS-associated enzymatic activity36. These data suggest that electrotaxis speed is independent of pathways involved in lifespan maintenance. The isp-1(qm150) and gas-1(fc21) also both activate the UPRMT73,74, which led us to investigate the role of atfs-1. We found that atfs-1 null mutant and gain-of-function mutant had a slower speed in our assay. Moreover, RNAi knock-down of atfs-1 reduced the speed of isp-1 mutant but not gas-1 mutant animals. Together with known roles of atfs-1 in regulating the expression of mitochondrial and cytosolic heat shock chaperons, these results support the conclusion that multiple stress response signaling affect the electrotaxis behavior of C. elegans.
The effect of ROS induced by PQ and mitochondrial mutants could also impact UPRER. Thus, we investigated the responses of animals treated with tunicamycin and observed defects in electrotaxis speed that were accompanied by an increase in the expression of the ER chaperone GRP78/BiP homolog hsp-4. Similar electrotaxis phenotypes were also observed in UPRER mutants, indicating that the ER genes play a crucial role in mediating normal movement behavior. The analysis of mutants affecting the three major arms of UPRER revealed that while ire-1, pek-1, and xbp-1 are needed to maintain a normal response, atf-6 is not essential. Differences between UPRER pathway components in mediating some of the biological processes have been reported earlier. Shen et al. (2001) found that while knockdown of pek-1 and ire-1 affected larval development, there was no such phenotype in the case of atf-675. Additionally, ire-1 and xbp-1 mutants have a short lifespan and slower development, but pek-1 and atf-6 mutants appear normal56,76. Finally, animals lacking ire-1, xbp-1, and atf-6 function show high sensitivity to pore forming bacterial toxin Cry5B but pek-1 does not appear to be involved46. In spite of these differences, we found that increasing the ER stress load by tunicamycin treatment exacerbated the electrotaxis defects of all three UPRER pathway mutants.
In addition to the known UPRER genes, we also tested the less well-characterized gene pqe-1. Mutations in pqe-1 are thought to cause proteotoxic ER stress, possibly due to the upregulation of global protein synthesis77. Consistent with this idea, our results showed that pqe-1 mutants have an abnormal electrotactic response. Studies in yeast have shown that the REXO1 (RNA exonuclease homolog 1) domain of PQE-1 functions in the processing of the 3′ ends of rRNA and tRNA78,79, which may suggest a role of pqe-1 in the regulation of protein translation. Since pqe-1 localizes to the cell nucleus23,24, it is possible that it affects genes whose products modulate the function of translation machinery. To investigate this further, we knocked-down the eIF4G homolog, ifg-1, a component of the initiation factor 4F (eIF4F) complex45, but saw no suppression of the pqe-1 mutant phenotype. This result combined with high sensitivity of mutant animals to tunicamycin and changes in the expression of multiple chaperons and xbp-1 lead us to suggest that pqe-1 plays an essential role in maintaining both HSR and organelle-specific stress response (UPR) pathways in C. elegans.
One of the ways by which stress can affect electrotaxis is by causing damage to neurons. Consistent with this, animals exposed to PQ and tunicamycin exhibited degenerated dopaminergic neurons (Fig. S1). A similar phenotype was also observed in pqe-1 mutants (Fig. S2). These findings, along with our previous work demonstrating the role of dopaminergic neuron signaling in mediating electrotaxis behavior22, led us to conclude that neuronal defects may in part contribute to the electrotaxis phenotype in animals as a result of stress-inducing conditions.
In addition to examining the effects of genetic mutations and compounds, we investigated whether environmental perturbations alter the electrotactic response. Among other effects , heat is reported to induce mitochondrial stress7. High temperatures have also been shown to modulate ER and cytosolic stress. This may involve multiple mechanisms, such as activation or repression of PERK depending on the severity of the heat regimen47 and changes in global protein synthesis and apoptosis47. We exposed animals to acute heat and found that their electrotaxis was unaffected. Chronic heat, however, severely reduced the speed, suggesting that behavior is affected by prolonged heat exposure, which is consistent with cellular stress playing a role in modulating electrotaxis. The resistance to heat-induced stress appears to depend on the transcription factor hsf-1, since hsf-1 mutants exhibit electrotaxis defects and are sensitive to heat treatments.
As diet can also play a role in the UPR maintenance53,80, we subjected worms to different forms of starvation. When C. elegans are starved during the L1 larval stage, they enter L1 arrest, which halts the development and reproductive growth, enhances stress resistance, modifies feeding behavior, and alters metabolic flux81,82. Starvation during the L4 larval and adult stages has beneficial effects such as extended life span, germline cell reduction, delayed reproduction, and thermotolerance83. At the cellular level, starvation induces both mitochondrial and ER stress53,80,84. Mitochondria become fragmented in starved animals and are cleared through mitophagy10,80,85. Zhang et al.86 reported that starvation caused ER stress and led to activation of PERK/eIF2α in an astrocyte cell line. Other groups have shown changes in the regulation of different ER proteins, such as chaperones84. Our work demonstrates that short-term DR does not affect the electrotactic response. However, eat-2 mutants that are chronically starved, and therefore mimic a long-term DR condition, show a significantly slower electrotaxis speed. It is conceivable that a reduction in speed may aid in the long life span of eat-2 mutants as the animals are slow feeders53. This line of reasoning agrees with previous findings that young adults lacking eat-2 function move slower on solid media33. Furthermore, eat-2 mutants show increased expression of hsp-453 but not hsp-654, suggesting higher ER stress levels.
The last stress condition we investigated was a daily exercise regimen. The benefits of exercise on muscular and neuronal health are well demonstrated. Healthier muscles through exercise allow animals to increase their athletic capacity63. At the molecular level, exercise activates the UPRER response as a protective mechanism87. Beneficial effects of exercise have also been demonstrated in C. elegans, such as a longer lifespan and improved neuronal and muscular health62,64,65,88. Laranjeiro et al. (2017) reported that the ER chaperone hsp-4 is downregulated in animals subjected to exercise, which suggests a conserved role of UPRER in exercise-induced benefits64. Our lab showed earlier that defects in muscles reduce the electrotaxis speed of C. elegans22. Consistent with the above studies, we found that daily exercise treatment significantly improved the electrotaxis speed of C. elegans and resulted in increased expression of heat shock chaperons as well as better preservation of mitochondrial morphology.
In summary, the work described in this paper shows that electrotactic movement of worms is reduced by mutations in genes that affect HSR, UPRMT, and UPRER as well as following chronic exposure to chemicals, heat, and reduced diet. In contrast, short bursts of daily exercise are beneficial since they resulted in a higher speed of animals and better preservation of mitochondrial morphology. Collectively, these findings lead us to conclude that conditions that elevate cellular stress for prolonged periods cause detrimental effects, whereas transient stress imparts a beneficial effect on health. The results established that a microfluidic-based assay is a reliable output of locomotory circuits and demonstrate that electrotaxis can be used to study stress response pathways in C. elegans.
Materials and methods
Strains and culturing
This study used the following C. elegans strains: N2: wild-type Bristol isolate; MQ887: isp-1(qm150); CW152: gas-1(fc21); TK22: mev-1(kn1); CY121: ucp-4(ok195); DY356: pink-1(ok3538); GA184: sod-2(gk257); GA186: sod-3(tm760); GA480: sod-2(gk257); sod-3(tm760); QC115: atfs-1(et15); VC3201: atfs-1(gk3094); RB545: pek-1(ok275); RB772: atf-6(ok551); RE666: ire-1(v33); SJ17 xbp-1(zc12) III; zcIs4 V; SJ4100: zcIs13(hsp-6::GFP); SJ4058: zcIs9(hsp-60::GFP); PS3551: hsf-1(sy441); DY693: pqe-1(ok1983);zcIs4 V; RB1611: pqe-1(ok1983); SJ4005: zcIs4(hsp-4::GFP); DY542: pqe-1(ok1983); bhEx138[pGLC72(Cel-dat-1p::YFP)]; CL2070: dvIs70[hsp-16.2::GFP + rol-6(su1006)]; DY353: bhEx138[pGLC72(Cel-dat-1p::YFP)]; SJ4103: zcIs14[myo-3::GFP(mit)] and DY356 (5x outcrossed RB2547): pink-1(ok3538). RB2547 and all other strains were originally obtained from the Caenorhabditis Genetics Center (University of Minnesota, St. Paul, MN)89.
Except where indicated, animals were grown and maintained at 20 °C on nematode growth medium (NGM) agar plates containing E. coli OP50 culture using previously described methods15. All experiments used age-synchronous populations obtained by bleach treatment15. Experiments involving wild-type and untreated controls were done using young adults unless stated otherwise. Growth times for all animals bearing mutations or undergoing treatments that affect developmental rate were appropriately adjusted.
Assays and treatments
The following methods were used to analyze worms in this study. Additional details are provided in supplementary methods.
Chemical treatments
Paraquat dichloride was obtained from Sigma-Aldrich (St. Louis, MO, USA) and tunicamycin was obtained from Bioshop Canada Inc (Burlington, ON, CA). Paraquat treatments were first prepared as 20 × solutions in M9 buffer; subsequently, 1 × treatment plates were produced by spreading 500 μL of the 20 × solutions across the surface of plates containing 10 mL of NGM agar. Tunicamycin treatments were first prepared as 5 mg/mL stock solutions in 100% DMSO, then diluted with water to make 20 × solutions; subsequently, 1 × treatment plates were produced by spreading 500 μL of the 20 × solutions across the surface of plates containing 10 mL of NGM agar. For chemical treatments, synchronized worms were grown on chemical-containing plates from L1 until adulthood. Young day-1 adults, based on direct observation, were used for experiments.
Heat stress
Animals were first grown at 20 °C until young adulthood. Plates were then sealed with parafilm and exposed to specific temperatures in a water bath for desired durations. Control plates were also sealed but kept at 20 °C. After the treatment, parafilm was removed and animals were allowed to recover at 20 °C for 30 min to an hour before electrotaxis assays. For 28 °C treatment, synchronized larvae were allowed to grow at 20 °C till day-1 adulthood and then shifted to 28 °C for 3 days.
Feeding bacterial and fungal cultures
C. elegans plates were seeded with overnight grown cultures of microorganisms. For E. coli, plant pathogens and fungus, L1 animals were transferred on plates and allowed to grow till adulthood. Day-1 adults were used for electrotaxis assays. In the case of Streptomyces venezuelae and Bacillius thuringiensis, L1 larvae were first fed with E. coli OP50 till adulthood. One day-old adults were transferred on maltose yeast extract medium (MYM) plates containing culture of S. venezuelae for 24 h. Bacillus thuringiensis culture was grown on NGM agar and fed to one day-old adults for 24 h. Electrotaxis was performed the following day. Control worms were grown in the presence of OP50 culture on NGM-Agar and MYM-Agar plates.
Starvation
Animals were first grown on OP50 until day-1 adulthood. They were then transferred to an unseeded NGM agar plate (no food) for 8 h to mimic acute starvation. Following the starvation period, electrotaxis assay was performed.
Exercise
Worms were subjected to exercise starting the L3 larval stage until day-6 adulthood (for a total of 7 days). Two different treatments were performed, each of which consisted of 30 min of daily swimming, followed by recovery on LB-agar plates. The first exercise regimen involved a belly dancer shaker in which worms, suspended in M9, were subjected to 100 rpm rotation. The second regimen consisted of keeping worms in M9 on the countertop without shaking, thereby allowing them to perform natural swimming.
Microchannel fabrication and electrotaxis assay
Microfluidic channels were fabricated as previously described21,90. The channel design was printed on a transparency sheet using high-resolution photoplotting to create a photomask, which was then used in conjunction with SU-8 100 negative photoresist (MicroChem Corp., MA, USA) to lithographically pattern the design onto a silicon wafer. Microchannels were then casted by pouring polydimethylsiloxane (PDMS) pre-polymer (Sylgard 184 Kit, Dow Corning Corp., MI, USA; 10:1 ratio of base and cross-linker) onto the resultant master mold and allowing 24 h for curing. The channel was then excised from the PDMS replica and fluid access ports were punched into each end. Next, the channel, a blank PDMS strip and a glass slide were oxidized via exposure to oxygen plasma for 40 s at 40 W power and stuck together to seal the microchannel. Lastly, plastic tubing and insulated copper wire were affixed to the punched reservoirs and secured with PDMS pre-polymer.
The electrotaxis assay protocol has also been described21,90. A syringe was attached to one of the inlet/outlet tubes of the PDMS microchannel to facilitate worm loading at the other tube. A power supply was connected via insulated copper wiring to the electrodes of the microchannel device to provide worms with electrical stimulus. A microscope, camera and monitor allowed visualization and recording of the electrotaxis experiment.
In preparation for the assay, worms were washed off of their culture plates, cleaned, and suspended in M9 buffer. Animals were then aspirated into the channel using the syringe pump. Both tubes were then laid flat at the same elevation to eliminate pressure-induced flow. Next, a 3 V/cm DC electric field was applied, and the worm’s resultant behaviour recorded by camera.
Electrotaxis was carried out for up to 5 min. Animals were allowed to travel a minimum distance of 5 mm in one direction, towards the cathode, after which the field polarity was reversed to induce a turning response. Locomotory data was extracted from recorded videos using custom MATLAB-based worm tracking software. Electrotaxis speed data was plotted in box plots.
Fluorescence microscopy and quantification
Animals were mounted on 2% agar pad containing glass slides. Before placing the cover slip, they were anesthetized using a 15 μL drop of 30 mM NaN3 mixed with a drop of M9. GFP fluorescence was visualized using a Zeiss Observer Z1 microscope equipped with an Apotome 2 and X-Cite R 120LED fluorescence illuminator. Fluorescence intensity was quantified using NIH ImageJ (http://rsbweb.nih.gov/ij/). Neurodegeneration was manually scored by counting the number of cell bodies of DAergic neurons and by assessing neurite morphologies and trajectories. Worms consist of two pairs of cephalic neurons (CEPs), a pair of anterior deirid neurons (ADE) and one pair of posterior dieirid neurons (PDE).
RNAi
The atfs-1 RNAi plasmid (pGLC171) was constructed by inserting a 759 bp genomic fragment into the L4440 vector using the restriction enzymes KpnI and XbaI. The fragment was obtained by PCR using the forward primer GL1649 (5'-AAGGGTACCCACTACTTGGAGAGCGACGAC-3') and reverse primer GL1650 (5'-AAGTCTAGACTACTTCTTGGAACTCCCTGC-3'). We used the RNAi gene silencing protocol described by Wu et al.41 Since authors reported that knock-down in parental isp-1 mutant worms caused an arrest of F1 larvae, L4-stage animals were treated with RNAi and day-2 old adults were analyzed. Synchronized worms were grown on OP50 until L4 stage and then transferred to RNAi plates containing either L4440 empty plasmid or atfs-1 RNAi plasmid. atfs-1 knock-down was confirmed by qPCR, which showed a range of ~ 2 × to 10 × reduction in different batches. For the ifg-1 RNAi, animals were fed with bacteria from Ahringer library. In this RNAi paradigm synchronized animals were grown on OP50 until day-1 adult stage and then transferred to ifg-1 RNAi plates for 48 h and analyzed right after.
Quantitative reverse transcription PCR (RT-qPCR)
For RT-qPCR experiments, synchronized cultures were prepared by bleaching adult hermaphrodites twice in two successive generations to obtain highly synchronous cultures. After the second round of bleaching, eggs were grown until the day-1 adulthood. Total RNA was extracted from these animals using trizol (Catalog Number T9424, Sigma-Aldrich, Canada), according to the manufacturer’s instructions. The SensiFAST cDNA Synthesis Kit (Catalog Number BIO-65053, MeridianBioscience, Canada) was used to obtain cDNA according to manufacturer’s instructions. RT-qPCR was performed (in triplicate) using the Bio-Rad cycler CFX 96 machine. The primers are listed in the Supplementary table (Table S1). The reaction was set up using SensiFAST SYBR Green Kit (Catalog Number BIO-98005, BIOLINE, USA) according to the manufacturer’s instructions. The expression levels of the analyzed genes were normalized to pmp-3.
Data analysis
GraphPad Prism 7 was used to plot the graphs. For all assays, data from repeat experiments were pooled and analyzed together. Statistical analyses were done using GraphPad Prism 7 except for RT-qPCR experiments that were analyzed using CFX Maestro 3.1 software (Bio-Rad, Canada; https://www.bio-rad.com/en-ca/product/cfx-maestro-software-for-cfx-real-time-pcr-instruments). P values less than 0.05 were considered statistically significant.
Supplementary Information
Acknowledgements
We thank Baekjun Sung for help with initial data collection, Devon Jones for adapting and fixing microfluidic channels, Sangeena Salam for some of the early experimental ideas and designs and Meghan Pepler for supplying the Streptomyces venezuelae culture. Some of the C. elegans strains were obtained from the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We also acknowledge the C. elegans Deletion Mutant Consortium for generating the deletion strains used in this study and the Bacillus Genetics Stock Center for providing the Bacillus thuringiensis culture. This work was supported by the NSERC Discovery Grant to BG and a joint CIHR CHRP award to PRS, BG and RKM.
Author contributions
B.G. conceived and supervised the project. S.K.B.T., M.H.M., and J.T. performed the experiments and analyzed data. B.G., S.K.B.T., and J.T. prepared the outline. B.G., S.K.B.T., J.T. and M.H.M. wrote the manuscript. S.K.B.T. and M.H.M. created the figures. P.R.S. and R.M. provided input throughout the project and reviewed the manuscript.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). Additional details are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shane K. B. Taylor and Muhammad H. Minhas.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-82466-z.
References
- 1.Fink G. In retrospect: eighty years of stress. Nature. 2016;539:175–176. doi: 10.1038/nature20473. [DOI] [PubMed] [Google Scholar]
- 2.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1038/138032a0. [DOI] [PubMed] [Google Scholar]
- 3.Yoshino J, Morikawa RK, Hasegawa E, Emoto K. Neural circuitry that evokes escape behavior upon activation of nociceptive sensory neurons in drosophila larvae. Curr. Biol. 2017;27:2499–2504.e3. doi: 10.1016/j.cub.2017.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Ladd CO, et al. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 2000;122:81–103. doi: 10.1016/S0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS. Mood disorders and allostatic load. Biol. Psychiat. 2003;54:200–207. doi: 10.1016/S0006-3223(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi-Sanabria R, Frankino PA, Paul JW, Tronnes SU, Dillin A. A futile battle? Protein quality control and the stress of aging. Dev. Cell. 2018;44:139–163. doi: 10.1016/j.devcel.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 2018;19:109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- 9.Fiorese CJ, et al. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr. Biol. 2016;26:2037–2043. doi: 10.1016/j.cub.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melber A, Haynes CM. UPR mt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remondelli P, Renna M. The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front. Mol. Neurosci. 2017;10:187. doi: 10.3389/fnmol.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuru A, Imai Y, Saito M, Kohno K. Novel mechanism of enhancing IRE1α-XBP1 signalling via the PERK-ATF4 pathway. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep24217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salminen A, Kaarniranta K. ER stress and hormetic regulation of the aging process. Ageing Res. Rev. 2010;9:211–217. doi: 10.1016/j.arr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner S. The genetics of caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corsi AK, Wightman B, Chalfie M. A transparent window into biology: a primer on Caenorhabditis elegans. Genetics. 2015;200:387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gourgou E, Chronis N. Chemically induced oxidative stress affects ASH neuronal function and behavior in C. elegans. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep38147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S, et al. Mutation of hop-1 and pink-1 attenuates vulnerability of neurotoxicity in C. elegans: the role of mitochondria-associated membrane proteins in Parkinsonism. Exp. Neurol. 2018;309:67–78. doi: 10.1016/j.expneurol.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C. A regulated response to impaired respiration slows behavioral rates and increases lifespan in caenorhabditis elegans. PLoS Genet. 2009;5:e1000450. doi: 10.1371/journal.pgen.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta, B. P. & Rezai, P. Microfluidic approaches for manipulating, imaging, and screening C. elegans. Micromachines7 (2016). [DOI] [PMC free article] [PubMed]
- 21.Rezai P, Siddiqui A, Selvaganapathy PR, Gupta BP. Electrotaxis of Caenorhabditis elegans in a microfluidic environment. Lab Chip. 2010;10:220–226. doi: 10.1039/B917486A. [DOI] [PubMed] [Google Scholar]
- 22.Salam S, et al. A microfluidic phenotype analysis system reveals function of sensory and dopaminergic neuron signaling in C. elegans electrotactic swimming behavior. Worm. 2013;2:e24558. doi: 10.4161/worm.24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faber PW, Voisine C, King DC, Bates EA, Hart AC. Glutamine/proline-rich PQE-1 proteins protect Caenorhabditis elegans neurons from huntingtin polyglutamine neurotoxicity. Proc. Natl. Acad. Sci. USA. 2002;99:17131–17136. doi: 10.1073/pnas.262544899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada K, Tsuchiya JI, Iino Y. Mutations in the pqe-1 gene enhance transgene expression in Caenorhabditis elegans. G3 Genes Genomes Genet. 2012;2:741–751. doi: 10.1534/g3.112.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumsta C, Thamsen M, Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antioxid. Redox Signal. 2011;14:1023–1037. doi: 10.1089/ars.2010.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr. Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz-Ortiz MA, et al. Curcumin enhances paraquat-induced apoptosis of N27 mesencephalic cells via the generation of reactive oxygen species. Neurotoxicology. 2009;30:1008–1018. doi: 10.1016/j.neuro.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XF, Thompson M, Xu YH. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab. Investig. 2016;96:496–507. doi: 10.1038/labinvest.2015.161. [DOI] [PubMed] [Google Scholar]
- 30.Chinta SJ, et al. Coupling endoplasmic reticulum stress to the cell death program in dopaminergic cells: effect of paraquat. NeuroMol. Med. 2008;10:333–342. doi: 10.1007/s12017-008-8047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper JF, et al. Activation of the mitochondrial unfolded protein response promotes longevity and dopamine neuron survival in Parkinson’s disease models. Sci. Rep. 2017;7:1–16. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA. 2015;112:E277–E286. doi: 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jafari G, et al. Tether mutations that restore function and suppress pleiotropic phenotypes of the C. elegans ISP-1(qm150) Rieske iron-sulfur protein. Proc. Natl. Acad. Sci. USA. 2015;112:E6148–E6157. doi: 10.1073/pnas.1509416112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollins JA, Howard AC, Dobbins SK, Washburn EH, Rogers AN. Assessing health span in Caenorhabditis elegans: lessons from short-lived mutants. J. Gerontol. Ser. A. Biol. Sci. Med. Sci. 2017;72:473–480. doi: 10.1093/gerona/glw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng J, Bussière F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell. 2001;1:633–644. doi: 10.1016/S1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 37.Hartman PS, Ishii N, Kayser EB, Morgan PG, Sedensky MM. Mitochondrial mutations differentially affect aging, mutability and anesthetic sensitivity in Caenorhabditis elegans. Mech. Ageing Dev. 2001;122:1187–1201. doi: 10.1016/S0047-6374(01)00259-7. [DOI] [PubMed] [Google Scholar]
- 38.Sedensky MM, Morgan PG. Mitochondrial respiration and reactive oxygen species in C. elegans. Exp. Gerontol. 2006;41:957–967. doi: 10.1016/j.exger.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 39.Van Raamsdonk, J. M. & Hekimi, S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet.5 (2009). [DOI] [PMC free article] [PubMed]
- 40.Bennett CF, et al. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat. Commun. 2014;5:1–10. doi: 10.1038/ncomms4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, et al. Mitochondrial unfolded protein response transcription factor ATFS-1 promotes longevity in a long-lived mitochondrial mutant through activation of stress response pathways. BMC Biol. 2018;16:147. doi: 10.1186/s12915-018-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauthan M, Ranji P, Pradenas NA, Pitot C, Pilon M. The mitochondrial unfolded protein response activator ATFS-1 protects cells from inhibition of the mevalonate pathway. Proc. Natl. Acad. Sci. USA. 2013;110:5981–5986. doi: 10.1073/pnas.1218778110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee A, et al. Unfolded protein response is required in nu/nu mice microvasculature for treating breast tumor with tunicamycin. J. Biol. Chem. 2011;286:29127–29138. doi: 10.1074/jbc.M110.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 45.Pan KZ, et al. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labbadia J, et al. Mitochondrial stress restores the heat shock response and prevents proteostasis collapse during aging. Cell Rep. 2017;21:1481–1494. doi: 10.1016/j.celrep.2017.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S, et al. Modulation of protein synthesis by eIF2α phosphorylation protects cell from heat stress-mediated apoptosis. Cells. 2018;7:254. doi: 10.3390/cells7120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell. 2004;15:657–664. doi: 10.1091/mbc.e03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunquell J, Morris S, Lu Y, Cheng F, Westerheide SD. The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genomics. 2016;17:559. doi: 10.1186/s12864-016-2837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapahi P, Kaeberlein M, Hansen M. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev. 2017;39:3–14. doi: 10.1016/j.arr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457:726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- 52.Robertson LT, Mitchell JR. Benefits of short-term dietary restriction in mammals. Exp. Gerontol. 2013;48:1043–1048. doi: 10.1016/j.exger.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matai L, et al. Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc. Natl. Acad. Sci. USA. 2019;116:17383–17392. doi: 10.1073/pnas.1900055116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray A, Martinez BA, Berkowitz LA, Caldwell GA, Caldwell KA. Mitochondrial dysfunction, oxidative stress, and neurodegeneration elicited by a bacterial metabolite in a C. elegans Parkinson’s model. Cell Death Dis. 2014;5:e984. doi: 10.1038/cddis.2013.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bischof, L. J. et al. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog.4 (2008). [DOI] [PMC free article] [PubMed]
- 57.Kwan, G., Charkowski, A. O. & Barak, J. D. Salmonella enterica suppresses Pectobacterium carotovorum subsp. carotovorum population and soft rot progression by acidifying the microaerophilic environment. MBio4 (2013). [DOI] [PMC free article] [PubMed]
- 58.Gohlke J, Deeken R. Plant responses to Agrobacterium tumefaciens and crown gall development. Front. Plant Sci. 2014;5:155. doi: 10.3389/fpls.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn S-J, Donahue K, Koh Y, Martin RR, Choi M-Y. Microbial-based double-stranded RNA production to develop cost-effective RNA interference application for insect pest management. Int. J. Insect Sci. 2019;11:117954331984032. doi: 10.1177/1179543319840323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Couillault C, Ewbank JJ. Diverse bacteria are pathogens of Caenorhabditis elegans. Infect. Immun. 2002;70:4705–4707. doi: 10.1128/IAI.70.8.4705-4707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE. 2009;4:e5886. doi: 10.1371/journal.pone.0005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laranjeiro R, et al. Swim exercise in Caenorhabditis elegans extends neuromuscular and gut healthspan, enhances learning ability, and protects against neurodegeneration. Proc. Natl. Acad. Sci. USA. 2019;116:23829–23839. doi: 10.1073/pnas.1909210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Invest. 2019;99:943–957. doi: 10.1038/s41374-019-0232-y. [DOI] [PubMed] [Google Scholar]
- 64.Laranjeiro R, Harinath G, Burke D, Braeckman BP, Driscoll M. Single swim sessions in C. elegans induce key features of mammalian exercise. BMC Biol. 2017;15:30. doi: 10.1186/s12915-017-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hartman, J. H. et al. Swimming exercise and transient food deprivation in Caenorhabditis elegans promote mitochondrial maintenance and protect against chemical-induced mitotoxicity. Sci. Rep.8 (2018). [DOI] [PMC free article] [PubMed]
- 66.Regmi SG, Rolland SG, Conradt B. Age-dependent changes in mitochondrial morphology and volume are not predictors of lifespan. Aging (Albany. NY) 2014;6:118–130. doi: 10.18632/aging.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim HM, Do CH, Lee DH. Taurine reduces ER stress in C. elegans. J. Biomed. Sci. 2010;17:1–6. doi: 10.1186/1423-0127-17-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang CL, Lee YC, Yang YC, Kuo TY, Huang NK. Minocycline prevents paraquat-induced cell death through attenuating endoplasmic reticulum stress and mitochondrial dysfunction. Toxicol. Lett. 2012;209:203–210. doi: 10.1016/j.toxlet.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 69.Kim-Han JS, Reichert SA, Quick KL, Dugan LL. BMCP1: a mitochondrial uncoupling protein in neurons which regulates mitochondrial function and oxidant production. J. Neurochem. 2008;79:658–668. doi: 10.1046/j.1471-4159.2001.00604.x. [DOI] [PubMed] [Google Scholar]
- 70.Krauss S, et al. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic β cell dysfunction. J. Clin. Invest. 2003;112:1831–1842. doi: 10.1172/JCI200319774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vidal-Puig AJ, et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- 72.Iser WB, Kim D, Bachman E, Wolkow C. Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans. Mech. Ageing Dev. 2005;126:1090–1096. doi: 10.1016/j.mad.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pujol C, Bratic-Hench I, Sumakovic M, Hench J, Mourier A. Succinate dehydrogenase upregulation destabilize complex i and limits the lifespan of gas-1 mutant. PLoS ONE. 2013;8:59493. doi: 10.1371/journal.pone.0059493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337:587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen X, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/S0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 76.Henis-Korenblit S, et al. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc. Natl. Acad. Sci. USA. 2010;107:9730–9735. doi: 10.1073/pnas.1002575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamada K, Tsuchiya JI, Iino Y. Mutations in the pqe1 gene enhance transgene expression in Caenorhabditis elegans. G3 Genes Genomes Genet. 2012;2:741–751. doi: 10.1534/g3.112.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozanick SG, et al. Rex1p deficiency leads to accumulation of precursor initiator tRNA Met and polyadenylation of substrate RNAs in Saccharomyces cerevisiae. Nucl. Acids Res. 2009;37:298–308. doi: 10.1093/nar/gkn925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Copela LA, Fernandez CF, Sherrer RL, Wolin SL. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA. 2008;14:1214–1227. doi: 10.1261/rna.1050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raut GK, Chakrabarti M, Pamarthy D, Bhadra MP. Glucose starvation-induced oxidative stress causes mitochondrial dysfunction and apoptosis via Prohibitin 1 upregulation in human breast cancer cells. Free Radic. Biol. Med. 2019;145:428–441. doi: 10.1016/j.freeradbiomed.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 81.Baugh LR. To grow or not to grow: Nutritional control of development during Caenorhabditis elegans L1 Arrest. Genetics. 2013;194:539–555. doi: 10.1534/genetics.113.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang C, You NJ, Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007;21:2161–2171. doi: 10.1101/gad.1573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, et al. Inhibition of starvation-triggered endoplasmic reticulum stress, autophagy, and apoptosis in ARPE-19 cells by taurine through modulating the expression of calpain-1 and calpain-2. Int. J. Mol. Sci. 2017;18:2146. doi: 10.3390/ijms18102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hibshman JD, et al. Nonselective autophagy reduces mitochondrial content during starvation in Caenorhabditis elegans. Am. J. Physiol. Cell Physiol. 2018;315:C781–C792. doi: 10.1152/ajpcell.00109.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang A, et al. EIF2α and caspase-12 activation are involved in oxygen-glucose-serum deprivation/restoration-induced apoptosis of spinal cord astrocytes. Neurosci. Lett. 2010;478:32–36. doi: 10.1016/j.neulet.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 87.Estébanez, B., De Paz, J. A., Cuevas, M. J. & González-Gallego, J. Endoplasmic reticulum unfolded protein response, aging and exercise: An update. Front. Physiol. 9 (2018). [DOI] [PMC free article] [PubMed]
- 88.Chuang HS, Kuo WJ, Lee CL, Chu IH, Chen CS. Exercise in an electrotactic flow chamber ameliorates age-related degeneration in Caenorhabditis elegans. Sci. Rep. 2016;6:1–11. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.The C. elegans deletion mutant consortium. Large-scale screening for targeted knockouts in the caenorhabditis elegans genome. G3 Genes, Genomes, Genet.2, 1415–1425 (2012). [DOI] [PMC free article] [PubMed]
- 90.Tong J, Rezai P, Salam S, Selvaganapathy PR, Gupta BP. Microfluidic-based electrotaxis for on-demand quantitative analysis of Caenorhabditis elegans’ locomotion. J. Vis. Exp. 2013 doi: 10.3791/50226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). Additional details are available from the corresponding author on reasonable request.