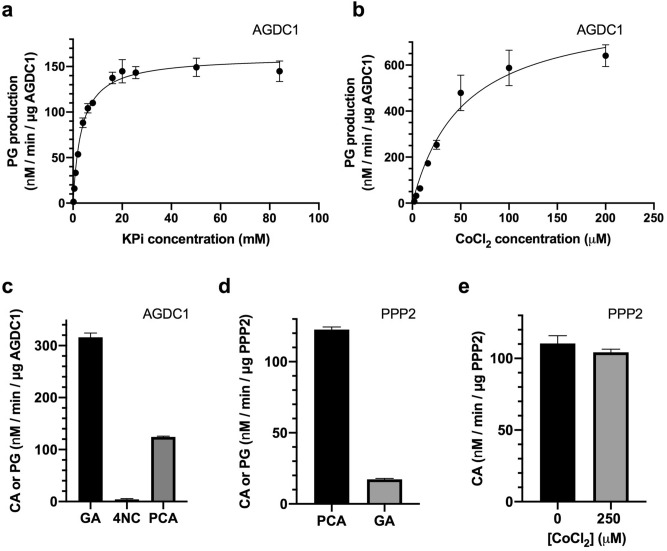
Figure 2.
Assay of wild-type purified AGDC1 and PPP2. (a) Effect of KPi, pH 6.5, on AGDC1 activity. The assay had 0.5 mM gallate (GA), 50 mM MES, pH 6.5, and 80 µg/ml AGDC1, and was performed at RT. (b) Effect of CoCl2 on AGDC1 activity. The assay had 0.5 mM GA, 50 mM KPi, pH 6.5, and 40 µg/ml AGDC1, and was performed at 37 ºC. To investigate CoCl2 activation, the background activity in the absence of CoCl2 was subtracted from the activity in the presence of CoCl2. (c) AGDC1 activity with 0.5 mM GA or protocatechuic acid (PCA). The inhibition by 0.5 mM of the substrate-analog 4-nitrocatechol (4NC) was checked in the presence of 0.5 mM GA. The assay had 50 mM KPi, pH 6.5, 250 µM CoCl2, and 80 µg/ml AGDC1, and was performed at RT. (d) Enzyme activity of PPP2 with GA and PCA. The activity assay was performed in the same conditions (50 mM KPi, pH 6.5, 160 µg/ml PPP2), at RT, with 0.5 mM PCA (black) or 0.5 mM GA (grey). (e) Effect of CoCl2 on PPP2 activity. The assay, performed at RT, had 50 mM KPi, pH 6.5, 0.5 mM PCA, and 160 µg/ml PPP2, with (grey) or without (black) 250 µM CoCl2. Consumption of GA was monitored at 259 nm, that of PCA at 250 nm. At the corresponding wavelengths, both PCA and GA had similar absorbance for 0.5 mM, in the same experimental conditions (i.e. same volume, same cuvette, same UV–VIS spectrometer). The addition of protein started the reaction. Error bars represent standard deviation from three independent measurements.