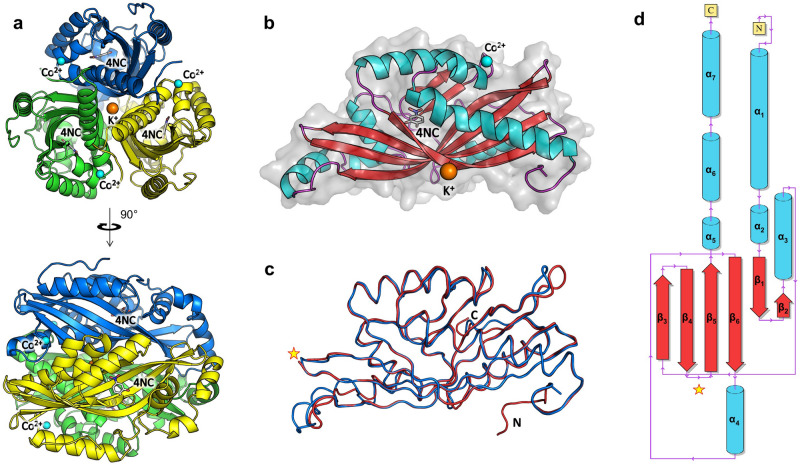
Figure 3.
The crystal structures of AGDC1 and PPP2. (a) The crystal structure of the 4NC/Co2+ complex of AGDC1 reveals a trimeric organization of the enzyme with a central potassium ion (orange sphere) and three peripheral cobalt binding sites (cyan spheres). The substrate analog 4-nitrocatechol (4NC) binds in a deep cleft, partially delimited by the β-barrel core. Each monomer is colored differently (blue, green, or yellow). (b) The monomer has an elongated shape, featuring an α + β fold, with a central β-barrel and peripheral helices. α-helices, β-strands, connecting loops, and the three-dimensional space-filling model are colored in cyan, red, purple, and light grey, respectively. 4NC is in ball-and-stick representation. (c) Superposition of the liganded structures of AGDC1 (blue) and PPP2 (red), in the same view as in (b). ‘N’ and ‘C’ indicate the N- and C-termini. The star indicates the equivalent position of the additional flexible loop in PPP2, with missing electron density. (d) Secondary structure topology diagram of AGDC1. The color scheme is as in (b).