Figure 1.
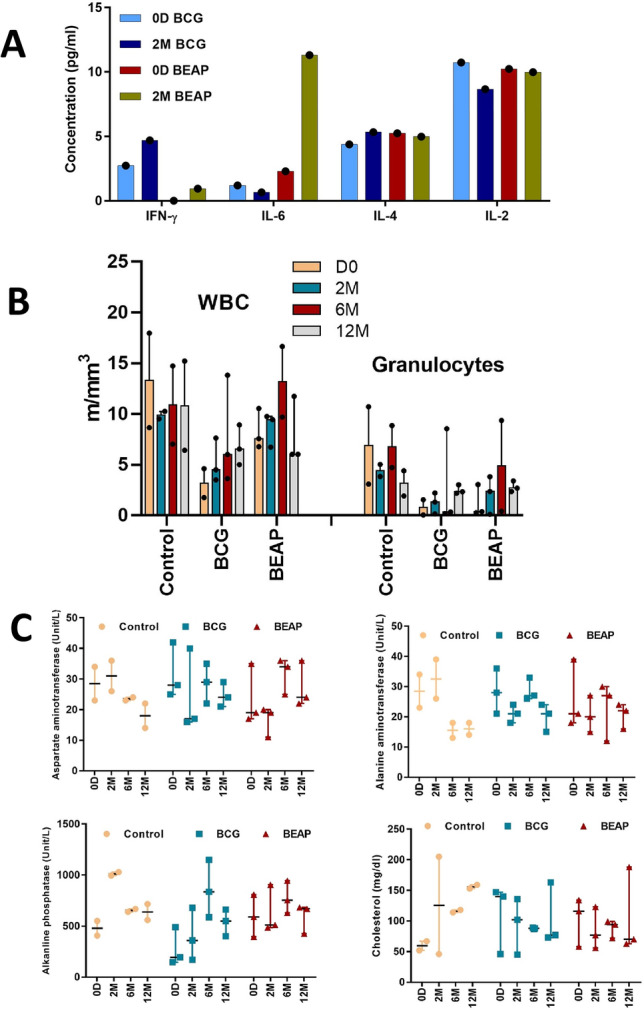
Evaluation of the toxicity or allergenicity of BEAP formulation. (A) Cytokine levels in the serum of BCG/BEAP immunized adult animal on D0 and at 2 months post immunization, (B) The granulocytes and WBC count in the control and post immunized infants. (C) Levels of liver secreted enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT) alkaline phosphatase (ALP) and cholesterol in the control and BCG/BEAP immunized infants at different time points. Dots indicate data points and bars are median with 95% confidence interval. Graphs were plotted using GraphPad Prism version-7 program (https://www.graphpad.com/).
