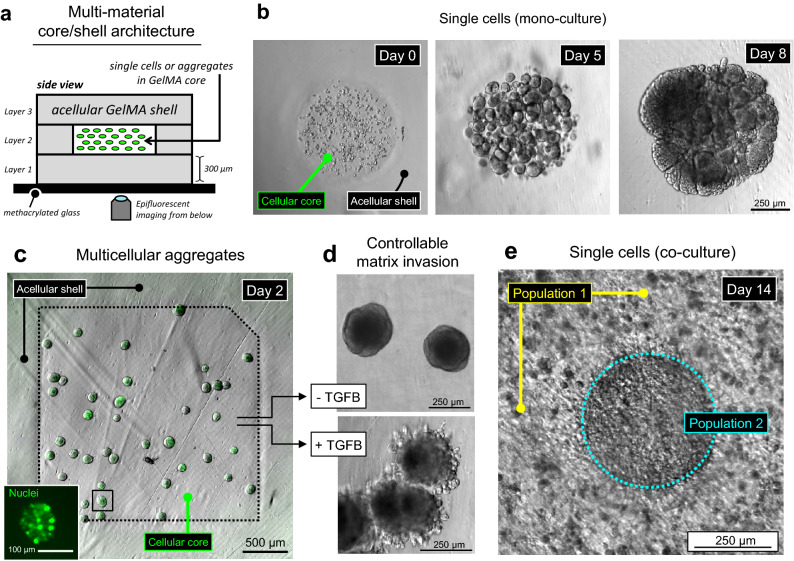
Figure 5.
Growth and functional response of bioprinted cells and multicellular aggregates. (a) Schematized core/shell architecture of multi-material bioprinted constructs (5 wt% GelMA), viewed from the side. The surrounding a cellular shell provides a large growth volume for 3D cell migration, preventing unwanted 2D cell spreading along the gel’s exterior surfaces. (b) Bioprinting of single 344SQ cells within sharply defined cylindrical cores. Due to the saline rinsing step between material selections, the surrounding a cellular shell is completely free of off-target cells. Over 5 days in culture, the encapsulated cells extensively proliferated and self-assembled into spheroids, a well-known behavior of 344SQ cells in other naturally derived hydrogel materials. By day 8, spheroids further coalesced into a dense unified structure (n = 3 gels). (c) Direct bioprinting of pre-formed multicellular aggregates. Inset: a GFP-tagged nuclear transcription factor enables single-cell visualization. (d) After several days, the aggregates cultured only in growth media maintained their original spherical morphologies, while aggregates exposed to hTGF-β1 adopted invasive morphologies. This controllable invasion behavior implicates the chosen architecture and GelMA bioink as a relevant system for deeper functional studies (n = 1 gel for each condition). (e) An idealized model of intratumoral heterogeneity. By day 14, the separate cell populations grew to form a dense and seamless regional interface, likely suitable for longitudinal interrogation of emergent tumormosaicism (n = 4 gels).