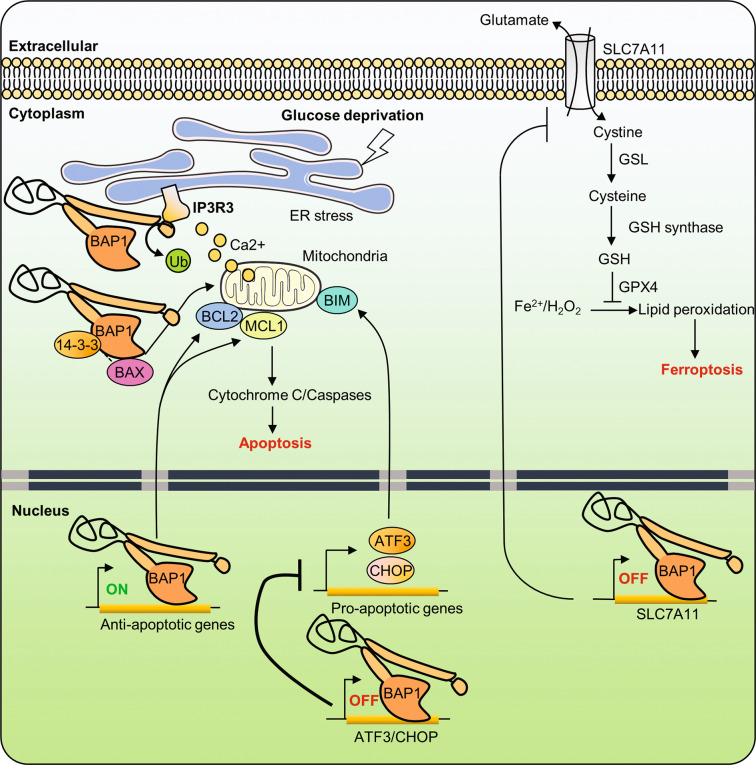
Fig. 3. Roles of the tumor suppressor BAP1 in cell death.
The tumor suppressor BAP1 stabilizes IP3R3 at the ER membrane through its DUB activity, which promotes the physiological release of Ca2+ into the cytoplasm and the mitochondria. Excessive Ca2+ release from ER results in high mitochondrial concentrations of Ca2+, which induces the release of cytochrome C, thus leading to apoptosis. BAP1 activity promotes the expression of pro-survival genes. BAP1 interaction with 14-3-3 releases BAX from 14-3-3 and promotes apoptosis. Conversely, loss of BAP1 induces apoptosis, in a cell-type-dependent manner, by inducing the transcriptional repression of pro-survival genes. On the other hand, BAP1 prevents apoptosis following glucose deprivation and ER stress by repressing the expression of proapoptotic factors. Finally, BAP1 promotes ferroptosis, a non-apoptotic form of cell death, by suppressing the expression of the SLC7A11 glutamate/cystine antiporter. Reduced expression of SLC7A11 leads to reduced levels of cystine uptake, which in turn leads to low levels of reduced GSH, therefore resulting in increased lipid peroxidation, which triggers ferroptosis. ATF3 activating transcription factor 3, CHOP C/EBP homologous protein, ER endoplasmic reticulum, GPX4 glutathione peroxidase 4, GSH reduced glutathione, GSL glutamate cysteine ligase, H2O2 hydrogen peroxide, iP3R3 inositol-1,4,5-triphosphate receptor, SLC7A11 solute carrier family 7 member 11, BAX BCL2-associated X, MCL1 myeloid cell leukemia 1.