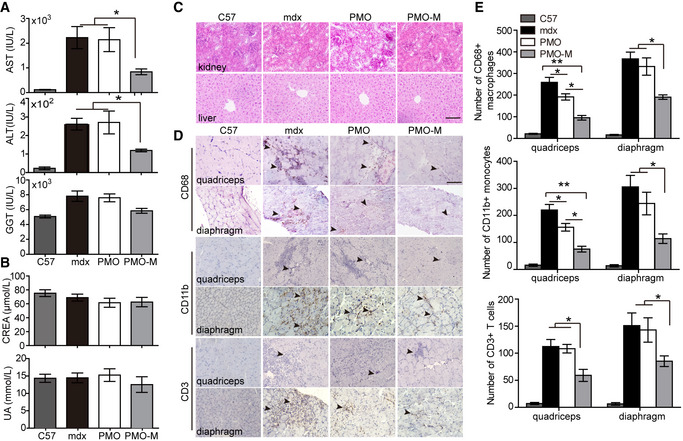
Figure 6. Evaluation of toxicology and inflammation in mdx mice treated with long‐term repeated doses of PMO‐M or PMO.
-
A, BMeasurement of serum indices including liver enzymes (AST, ALT, and GGT) (A) and kidney markers (CREA and UA) (B) from wild‐type C57BL/6 (C57) (n = 3), untreated mdx controls (mdx) (n = 3), and mdx mice treated with PMO‐M (n = 4) or PMO alone (n = 3) to reflect liver and kidney functions (*P < 0.05, one‐way ANOVA post hoc Student–Newman–Keuls test).
-
CMorphological examination of liver and kidney from wild‐type C57BL/6 (C57), untreated mdx controls (mdx), and treated mdx mice (scale bar: 100 μm).
-
D, EImmunohistochemistry (D) and quantification (E) of macrophages, T cells, and monocytes in quadriceps and diaphragm from wild‐type C57BL/6 (C57) (n = 3), untreated mdx controls (mdx) (n = 3), and mdx mice treated with PMO‐M (n = 4) or PMO (n = 3) (scale bar: 100 μm). The arrowheads point to CD68+ macrophages, CD3+ T cells, or CD11b+ monocytes (*P < 0.05, **P < 0.001, one‐way ANOVA post hoc Student–Newman–Keuls test).
Data information: Data were presented as mean ± sem. Exact P values are specified in Appendix Table S1.
