Fig. 4: Established stakeholder interaction channels.
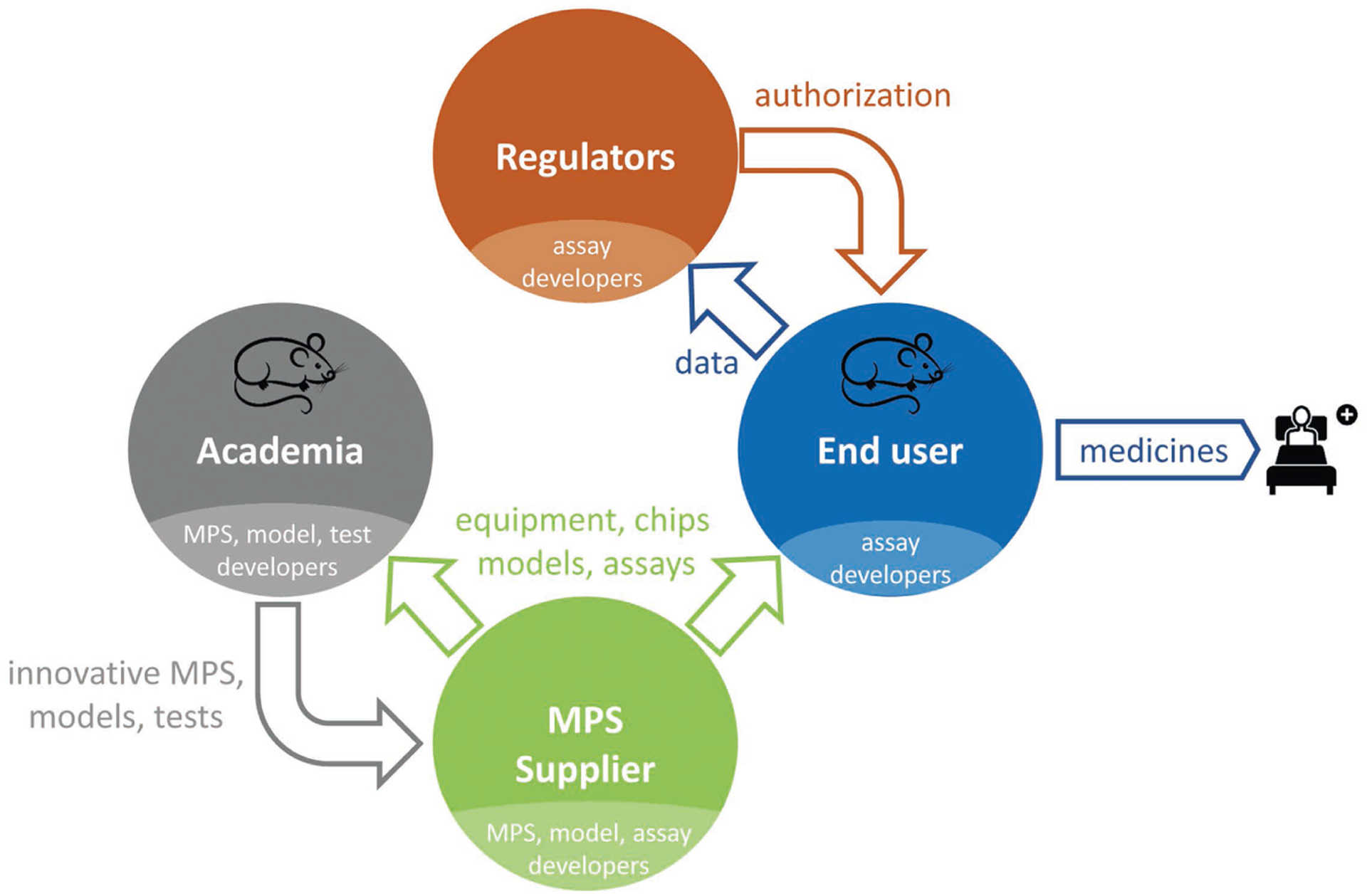
MPS devices, chips, models and methods are provided to end users and academia for data generation by the supplier industry. End users (pharmaceutical industry and CROs) are translating the methods into qualified assays for internal decision-making and use the data for clinical trial submissions, eventually resulting in authorization by regulators. Academia develops new MPS solutions that are absorbed by MPS suppliers. All four stakeholders consist of developers of MPS technologies.
