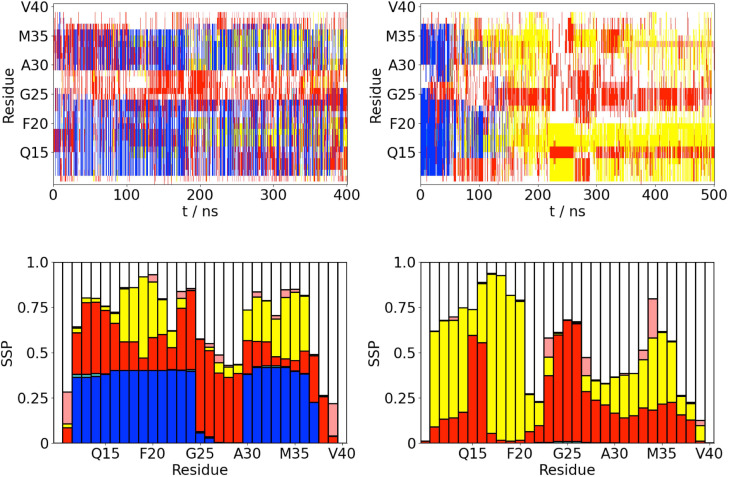
FIG. 1.
(a) Time variation of the secondary structure for A(10–40) at the AWI (left) and in the bulk solution (right). -helix, -strand, turn, 3/10-helix, and random coil (disordered) denoted by blue, yellow, red, cyan, and white, respectively. (b) Propensity for different secondary structure motifs (both ordered and random coil) for each residue (averaged over last 200 ns of simulations). -helix, -strand, turn, 3/10-helix, and random coil (disordered) denoted by blue, yellow, red, cyan, and white, respectively.