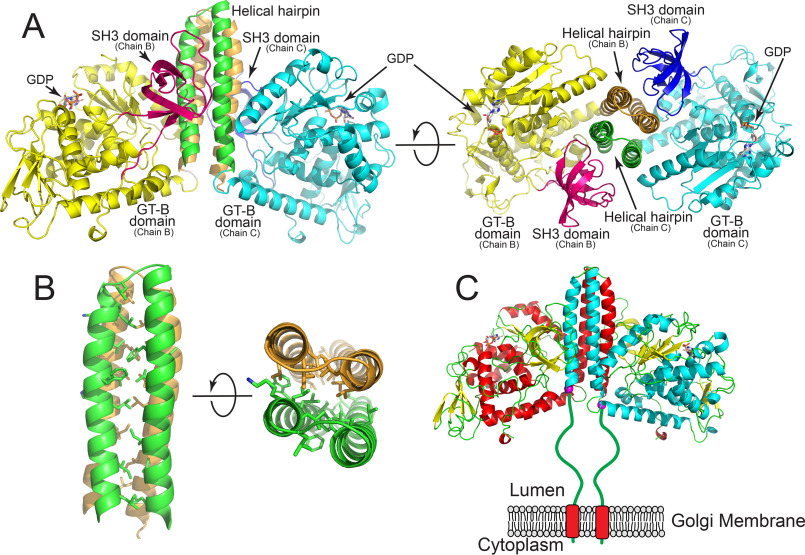
Figure 2.
Structure of the FUT8:GDP complex. The structure of the human FUT8:GDP dimer. The interface is formed from an extended 4-helix bundle with contributions from two helices of each chain and likely represents the biological dimer in solution. A, representation of the homodimer where discrete domains of the proteins are colored differently. Chain B is comprised of an N-terminal helix pair (tan) followed by the GT-B domain (yellow), and the C-terminal SH3 domain (magenta). For chain C, the N-terminal helical pair (green) is followed by the GT-B domain (cyan) and the SH3 domain (blue). Two rotations of the structure are shown illustrating the 4-helix bundle and the interactions between the SH3 domains and the side of the helical bundle. B, zoom-in representation of hydrophobic side chain interactions within the 4-helix bundle shown as a side view and end-on view. C, cartoon representation of the full-length biological dimer of FUT8 as a transmembrane, Golgi-localized enzyme in vivo with a 77-amino acid linker “stem region” between the transmembrane span and the catalytic domain (green line). Helices of chain B (red) and chain C (cyan) are shown in cartoon representation, β strands are shown as yellow cartoons and GDP molecules are shown as white sticks. The N-terminal ends of the respective chains in the crystal structure are shown as purple spheres.