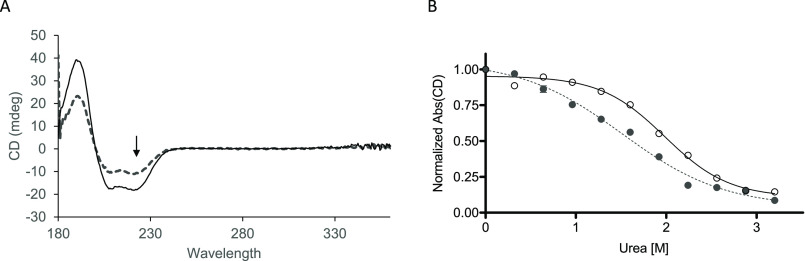
Figure 1.
Tropomyosin chemical unfolding in the presence of urea. A, uncorrected ellipticity of 2 μm M8R (dashed line) and WT (solid line) tropomyosin at 0 m urea shows typical peaks for α-helical coil (190, 200, and 220 nm). B, unfolding of tropomyosin, measured in ellipticity changes at 220 nm (see arrow in A), as chemical denaturant urea is added to buffer shows a difference in M8R tropomyosin and WT tropomyosin sensitivity. M8R (filled circles with dotted line) and WT (open circles with solid line) data are fit to a three-parameter Hill equation (CUrea-unfold at 1.52 ± 0.09 m versus WT 1.98 ± 0.05 m; nHUrea-unfold 0.77 ± 0.15 versus WT 1.23 ± 0.17).