Figure 5.
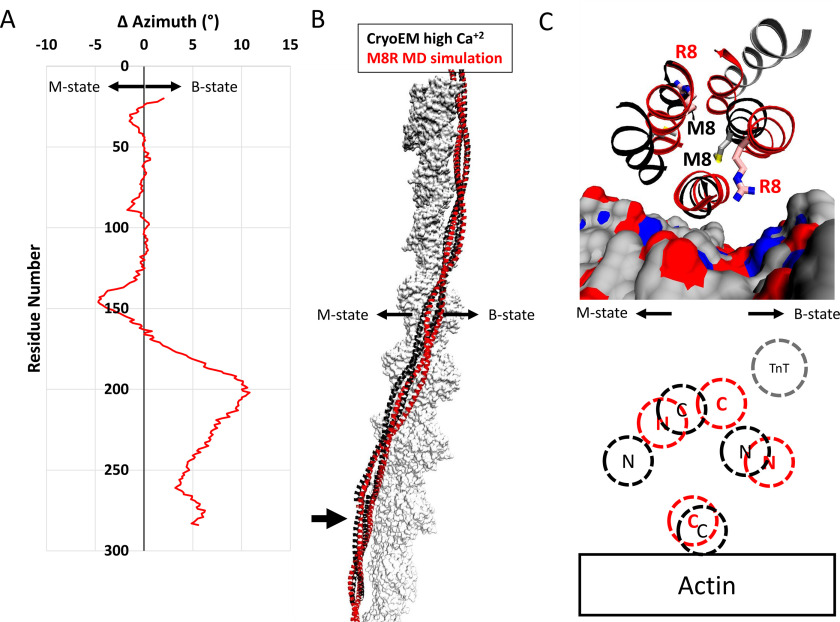
The alterations to the overlap domain caused by the M8R mutation result in a shift away from the closed state compared with the WT structure. A, change in azimuthal position by single residue pairs calculated by subtracting the azimuthal angle of the center of mass of the WT backbone atoms from the position of the M8R atoms. On average, the displacement of the tropomyosin dimer is ∼1° away from the closed/open states for the mutant. In the orientation used here, positive azimthual values represent motions toward the structural B-state, and negative azimuthal values represent motions toward the structural M-state. B, comparison of the WT refined high Ca2+ cryo-EM structure (black ribbon) and a representative structure of M8R mutant (red ribbon) on actin (gray surface) shows the differences in azimuthal positions calculated in A. C, close view of the structural changes of R8 on the end-to-end bond region of tropomyosin-on-actin colored as in B. Actin is rendered as surface-colored as in Fig. 3, and troponin T from the refined high Ca2+ cryo-EM structure is shown in gray ribbon. C, schematic diagram illustrating the relative positions of WT (black) and M8R (red) N and C termini on actin, and the position of troponin T N terminus binding (gray).