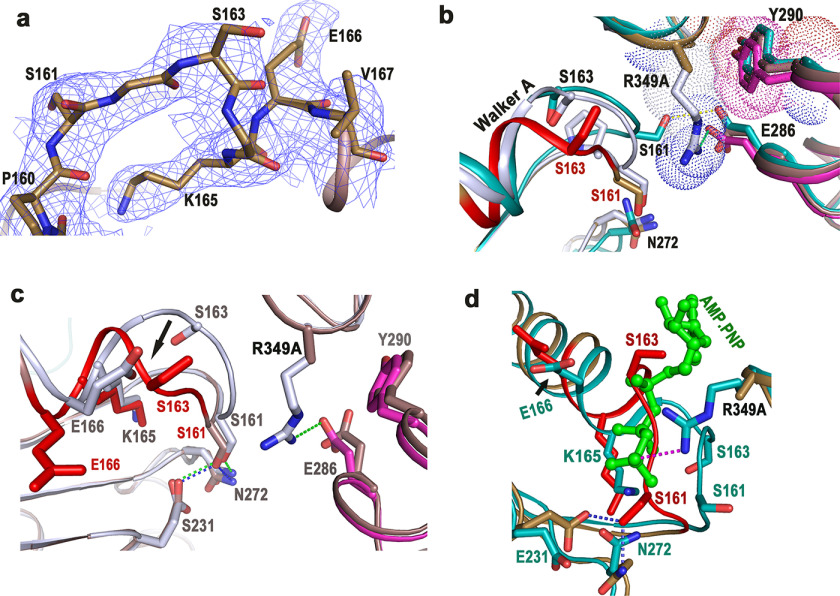
Figure 3.
ATP-binding site of FlrCC-R349A was obscured by Walker A. a, 2Fo − Fc electron density map, contoured at 1σ, around Walker A motif of FlrCC-R349A. b, superposition of Walker A regions of Nt-free and AMP-PNP–bound FlrCC on that of FlrCC-R349A. The mutation site is shown as R349A. Hydrophobic packing between cis-acting Arg349 (gray) with trans-acting Tyr290 (magenta) is shown in Nt-free FlrCC. Both protomers of AMP-PNP–bound FlrCC are shown in sea green. c, superposition of FlrCC-R349A (brown) on Nt-free FlrCC structure (gray) depicted the conformational difference in Walker A (shown by arrow). d, superposition of FlrCC-R349A on AMP-PNP–bound FlrCC (sea green) has shown encroachment of ATP-binding site by Walker A of the variant. Bound ATP is shown as green ball and stick. In b–d, Walker A of FlrCC-R349A is shown in red for clarity.