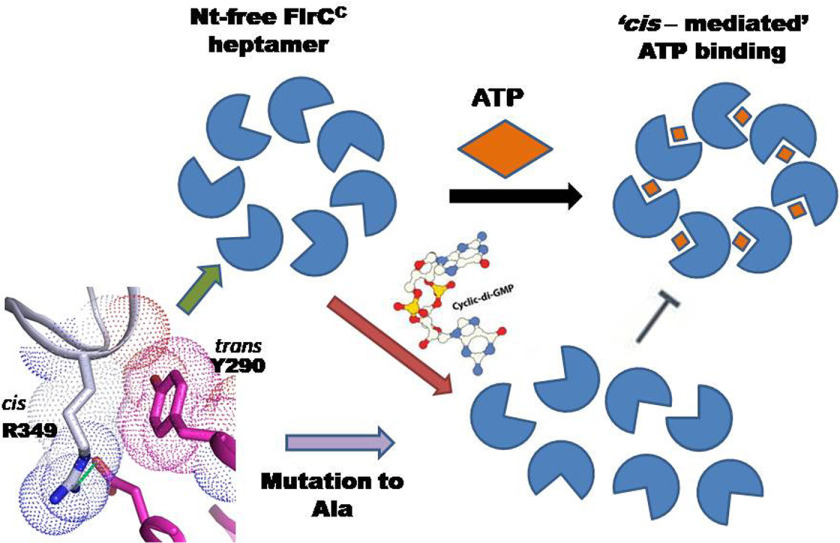
Figure 7.
Schematic representation of proposed mechanism. Hydrophobic packing of trans-acting Tyr290 with cis-acting Arg349 is pivotal in maintaining the heptameric state, which is required for ATPase activity. In the presence of c-di-GMP, FlrC loses heptameric state with abrogation of ATPase activity.