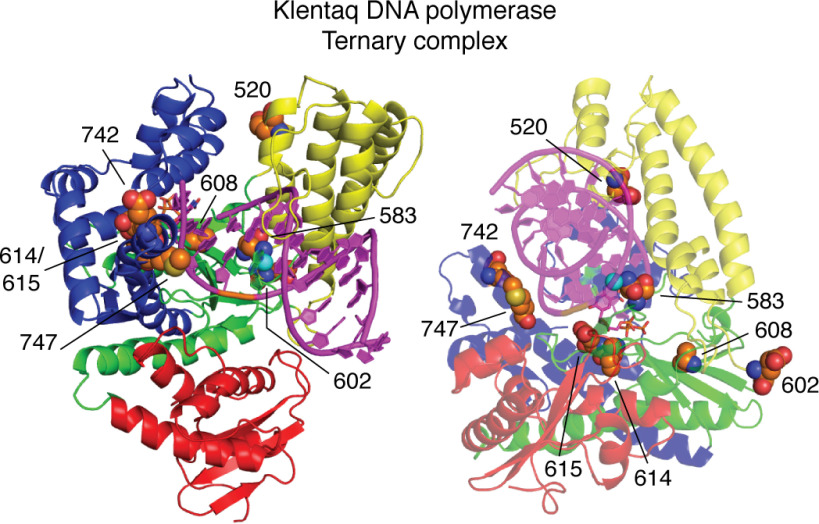
Figure 7.
A cartoon rendering showing two views of the ternary complex of Klentaq (fingers, blue; palm, green; thumb, yellow; exonuclease domain, red) with amino acid substitutions common to evolved Klentaq variants shown in van der Waals sphere renderings (carbon, orange; oxygen, red; nitrogen, blue; sulfur, yellow). None of the side chains of these residues are involved in direct hydrogen-bonding interactions with the template-primer or dNTP in the active site. Most of these residues are distant from substrate template-primer and dNTP, although the main-chain NH of Glu-615 hydrogen-bonds to the 3′-OH of the dNTP deoxyribose ring, and Asn-583 makes a water-mediated hydrogen bond to the backbone of the template strand. In addition, Ile-614 and Glu-615 are within van der Waals contact distance of the dNTP bound to the active site, and Met-747 is within contact distance of the template strand.