Figure 3.
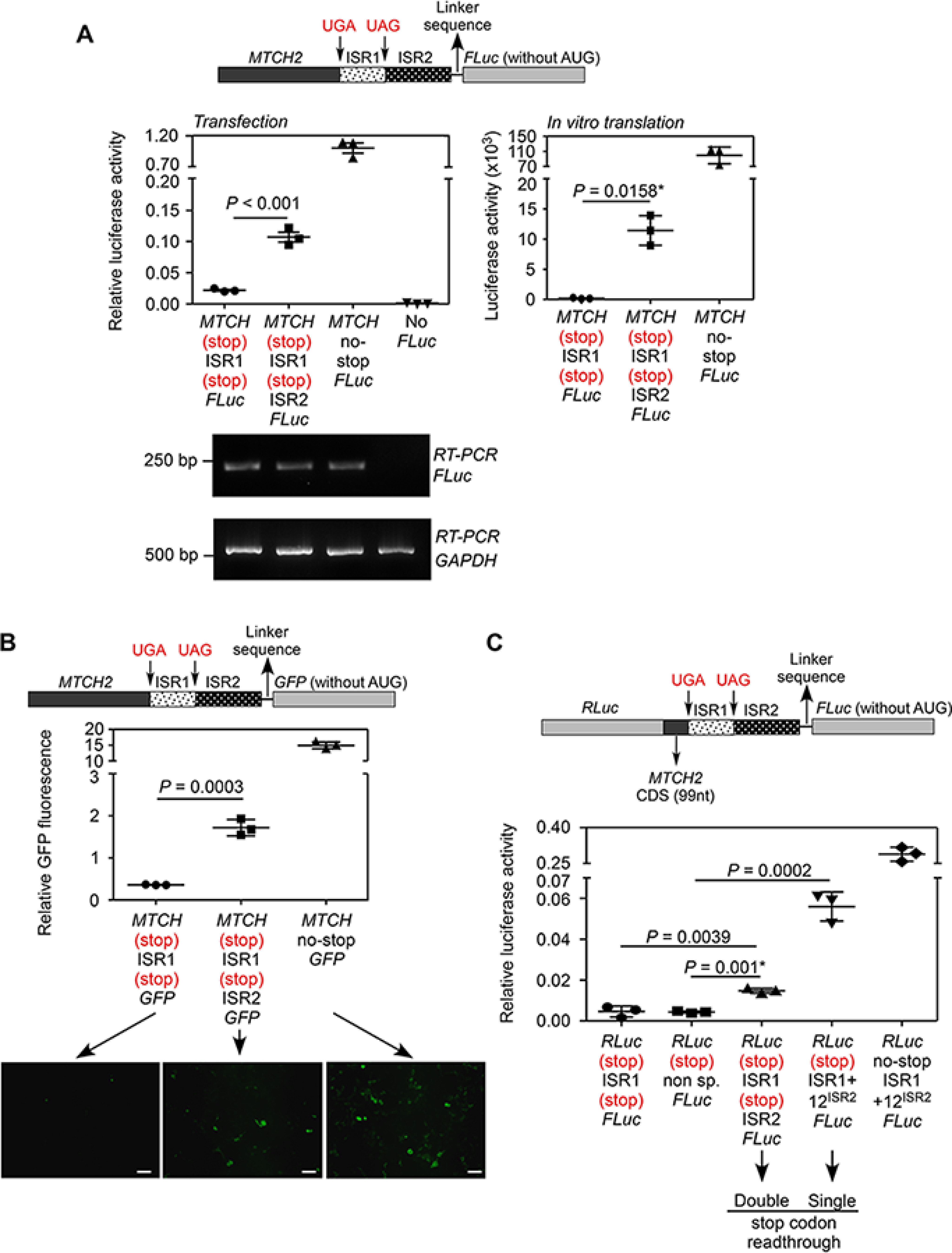
Demonstration of SCR across the canonical and the first in-frame stop codons of MTCH2 (double-SCR). A, luminescence-based assay: plasmid expressing in-frame MTCH2-(UGA)-ISR1-(UAG)-ISR2-FLuc and its variants were transfected in HEK293 cells. FLuc activity relative to the activity of co-transfected RLuc is shown (left). A construct without ISR2 served as negative control. RT-PCR of FLuc mRNA is also shown. The same constructs were subjected to in vitro transcription and in vitro translation using rabbit reticulocyte lysate. Luciferase activity is shown (right). B, fluorescence-based assay: HEK293 cells were transfected with constructs similar to the ones described in A, but the coding sequence of FLuc was replaced with that of GFP. Fluorescence can be observed only if there is double-SCR. Fluorescence was analyzed by flow cytometry (graph) and by fluorescence microcopy (images below). Scale bar, 50 μm. Graph shows the mean intensity of GFP fluorescence relative to that of co-transfected RFP fluorescence. C, dual luciferase-based assay: 99 nucleotides from the 3′ end of the MTCH2 coding sequence, ISR1 and ISR2 were cloned between the coding sequences of RLuc and FLuc (schematic). All were in-frame. Although RLuc activity is expected all the time, FLuc activity is expected only if there is double-SCR. This construct was transfected in HEK293 cells and FLuc activity relative to RLuc is shown. All graphs show mean ± S.D. (n = 3). Results are representatives of at least three independent experiments done in triplicate. Two-tailed Student's t test was used to calculate the p value. *, Welch's correction was applied. MTCH, MTCH2; CDS, coding sequence.
