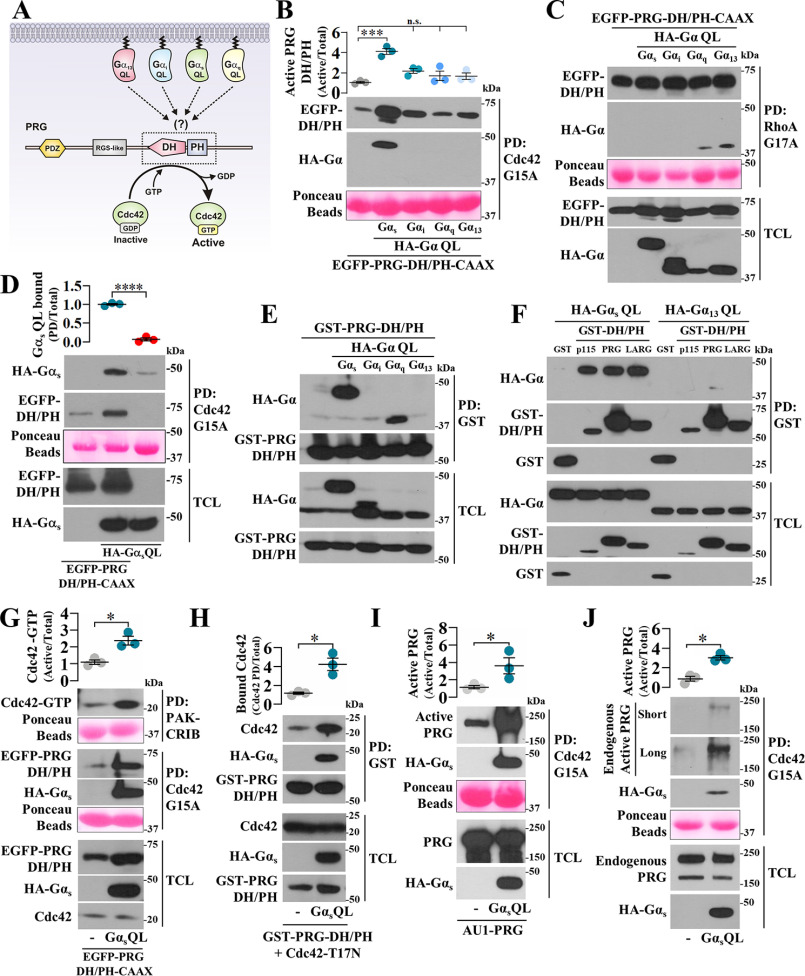
Figure 2.
Gαs-Q227L binds PRG DH/PH enabling this prototypical RhoA-specific GEF to directly activate Cdc42. A, hypothetic model postulating Gα subunits as potential regulators of PRG DH/PH catalytic module. B and C, the effect of GTPase-deficient Gα subunits on the interaction of EGFP-PRG-DH/PH-CAAX with Cdc42-G15A (B) and RhoA-G17A (C) was analyzed by pulldown (PD) using lysates from HEK293T cells transfected with HA-tagged Gαs, Gαi, Gαq, or Gα13 QL mutants and EGFP-PRG-DH/PH-CAAX. The graph in B represents the means ± S.E. (n = 3). ***, p < 0.001; n.s., no significance, one-way ANOVA followed Tukey. D, to address whether Gαs-QL detected in the PRG-DH/PH·Cdc42-G15A pulldown was part of a ternary complex, pulldown experiments were done in the presence or absence of PRG-DH/PH. The graph represents the means ± S.E. (n = 3). **, p < 0.0001, t test. E, the potential interaction between active Gα subunits and PRG DH/PH was analyzed in HEK293T cells transfected with GST-PRG-DH/PH and HA-tagged GTPase-deficient Gα subunits subjected to pulldown assays. F, interaction between Gαs-QL and the catalytic domain of the three RH-RhoGEFs was assayed by pulldown using HEK293T cells transfected with HA-Gαs-QL and GST-p115-DH/PH, GST-PRG-DH/PH, or GST-LARG-DH/PH. GST and HA-Gα13-QL served as negative controls. G, the effect of Gαs-QL on the activation of Cdc42 by PRG-DH/PH was assessed by pulldown using lysates of transfected HEK293T cells. The graph represents the means ± S.E. (n = 3). **, p = 0.01, t test. Representative blots show the fraction of active Cdc42 (top panel) and the active fraction of PRG-DH/PH with affinity for Cdc42-G15A (middle panel). H, the effect of Gαs-QL on the interaction between PRG-DH/PH and Cdc42-T17N was analyzed by pulldown using lysates of transfected HEK293T cells. The graph represents the means ± S.E. (n = 3). *, p = 0.01, t test. I and J, the effect of Gαs-QL on full-length PRG affinity for Cdc42 was analyzed in HEK293T cells that were transfected with full-length AU1-PRG (I) without or with HA-Gαs-QL or only with HA-Gαs-QL to address its effect on endogenous PRG (J). The active fraction of full-length PRG with affinity for Cdc42-G15A was isolated by pulldown and revealed by immunoblotting with anti-PRG antibodies. The graphs represents the means ± S.E. (n = 3). *, p = 0.01 in H and 0.04 in I, t test.