Abstract
Reactive oxygen species (ROS) are an unavoidable host environmental cue for intracellular pathogens such as Mycobacterium tuberculosis and Mycobacterium bovis; however, the signaling pathway in mycobacteria for sensing and responding to environmental stress remains largely unclear. Here, we characterize a novel CmtR-Zur-ESX3-Zn2+ regulatory pathway in M. bovis that aids mycobacterial survival under oxidative stress. We demonstrate that CmtR functions as a novel redox sensor and that its expression can be significantly induced under H2O2 stress. CmtR can physically interact with the negative regulator Zur and de-represses the expression of the esx-3 operon, which leads to Zn2+ accumulation and promotion of reactive oxygen species detoxication in mycobacterial cells. Zn2+ can also act as an effector molecule of the CmtR regulator, using which the latter can de-repress its own expression for further inducing bacterial antioxidant adaptation. Consistently, CmtR can induce the expression of EsxH, a component of esx-3 operon involved in Zn2+ transportation that has been reported earlier, and inhibit phagosome maturation in macrophages. Lastly, CmtR significantly contributes to bacterial survival in macrophages and in the lungs of infected mice. Our findings reveal the existence of an antioxidant regulatory pathway in mycobacteria and provide novel information on stress-triggered gene regulation and its association with host–pathogen interaction.
Keywords: Mycobacterium bovis, oxidative stress, ESX-3, zinc ion, CmtR, gene regulation, gene transcription, bacterial genetics, bacterial pathogenesis, microbiology, zinc, mycobacterium
Gene expression at accurate time points is of critical importance for the rapid adaptation of pathogens to harsh environments. Reactive oxygen species (ROS) are an unavoidable environmental stimuli for nearly all organisms. This particularly holds true for intracellular pathogens such as Mycobacterium tuberculosis (Mtb) and Mycobacterium bovis. During infection, the intracellular pathogen is exposed to a variety of host-mediated stresses, in which ROS is one of the primary antimicrobial agents produced by phagocyte oxidase in macrophages (1–4). Excess ROS can induce oxidative stress and, therefore, control bacterial infection by damaging essential cellular components such as proteins, lipids, and nucleic acids in bacteria (5). M. tuberculosis is one of the most persistent intracellular pathogens and possesses unique mechanisms to combat oxidative stress for survival in host cells. For example, Mtb cells have a thick wall (6) and contain mycothiol at millimolar concentrations (7, 8); in particular, Mtb encodes multiple protective enzymes such as catalase (KatG) (9), superoxide dismutases (10), and peroxynitrite reductase complex (11, 12). However, the molecular mechanism and the signaling pathways by which mycobacteria adaptively promote antioxidant regulation are yet to be elucidated.
Specific regulatory sensors have evolved in certain bacteria to detect different environmental cues, such as the presence or absence of oxygen or ROS (13). By binding different cofactors, these sensors can convert oxidative signals into regulatory outputs and further trigger bacterial adaptation to stress (13). For example, OxyR is a well-characterized redox sensor in Escherichia coli (14, 15). The cysteine residues in it can be oxidized to form an intramolecular disulfide bond and promote the katG expression (16). By sensing and responding to the presence of organic hydroperoxides, Bacillus subtilis OhrR regulates the expression of organic hydroperoxide reductase (ohrA) to support bacterial survival in toxic environments (17). In mycobacterial species, only redox-sensing regulators have been characterized clearly, of which MosR is a redox-dependent transcription factor similar to B. subtilis OhrR (18). In addition, OxyS has also been characterized as a redox sensor that regulates the expression of katG (19).
In recent times, a growing body of evidence has suggested that metal ions, including iron, manganese, and even zinc ions, can function as essential structural or catalytic cofactors for activating antioxidant enzymes in certain bacteria (20–24). ESX-3 is conserved in several mycobacterial species and is one of the five type-VII secretion systems for exporting proteins linked to tuberculosis pathogenesis (25, 26). EsxH, which is one of the components encoded by the esx-3 operon, can target the host endosomal sorting complex to impair phagosome maturation and the recognition of Mtb-infected cells (27, 28). The EsxG-EsxH heterodimer can induce the host immunopathologic response and improve bacterial survival during infection (29, 30). Notably, the expression of the esx-3 operon is regulated by the zinc uptake repressor (Zur) and the iron-dependent transcriptional repressor (IdeR) to maintain zinc and iron homeostasis, respectively, in M. tuberculosis (31–33). The EsxG-EsxH complex contains a specific Zn2+ binding site formed of a cluster of histidine residues in EsxH (34) and has been linked to adaptations of M. tuberculosis under low zinc-concentration environments. The expression and secretion of the EsxG-EsxH complex have been observed to significantly affect the interaction between M. tuberculosis and macrophages, as well as bacterial survival in host cells (35). However, the roles of Zn2+ in mycobacterial antioxidant defense and those of ESX-3 in the maintenance of metal homeostasis remain unclear. The mechanism by which a redox sensor is potentially linked to the expression of the esx-3 operon, if it is linked at all, and its effect on bacterial survival under oxidative stress as well as within the host remain unexplored.
In this study, we report that CmtR, usually regarded as a Cd/Pb sensor (36–38), is a novel redox sensor in M. bovis that is essential for mycobacterial growth under oxidative stress. Upon sensing an oxidative stress signal, CmtR can trigger the expression of the esx-3 operon by physically interacting with Zur to de-repress its activity, which affects Zn2+ homeostasis in bacterial cells and enhances bacterial antioxidant adaptation. Meanwhile, Zn2+ can also act as an effector molecule of the CmtR regulator to further promote the antioxidant regulation pathway. Lastly, CmtR was observed to significantly contribute to bacterial survival during infection. Our findings indicate the existence of a novel oxidative stress-triggered signaling circuit and provide new insights into mycobacterial adaptation to the host environment during infection.
Results
CmtR enhances the resistance of M. bovis BCG to H2O2
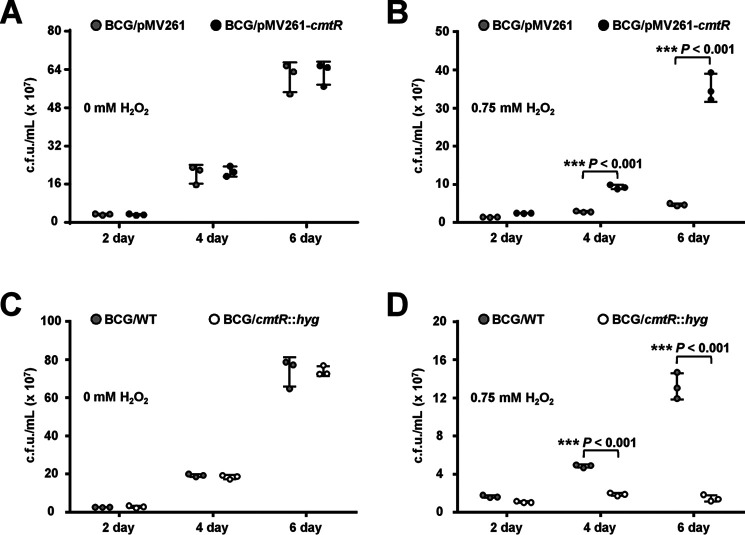
In a previous screening of the transcriptional factor library of M. tuberculosis, we preliminarily characterized CmtR as a potential regulator that contributes to H2O2 resistance of M. bovis BCG (Fig. S1A). In this study, we further determined the effects of the varying expression levels of cmtR on mycobacterial growth under H2O2 stress. As shown in Fig. 1, when comparing the growth of two mycobacterial strains treated with 0.75 mm H2O2, the bacterial counts of the cmtR overexpressing M. bovis were significantly higher than those of the WT strain at 4 and 6 days (Fig. 1B), which indicates that cmtR overexpression enhanced the resistance of M. bovis to H2O2. In contrast, the cmtR-deleted strain was more sensitive to H2O2 than the WT strain (Fig. 1D). In the absence of H2O2 stress, no obvious growth difference was observed between the WT strain and the overexpressing or deletion strains (Fig. 1, A and C). These results indicate that cmtR expression enhances the resistance of M. bovis BCG to H2O2.
Figure 1.
Assays for the regulatory effect of CmtR on H2O2 resistance of M. bovis BCG strains. A and B, comparative assays for the growth difference between BCG/pMV261 (control) and BCG/pMV261-cmtR (cmtR overexpression) strains in 7H9 medium (A) and in medium supplemented with 0.75 mm H2O2 (B). C and D, comparative assays for the growth difference between BCG/WT (control) and BCG/cmtR::hyg (cmtR-deleted) strains grown in 7H9 medium (C) and in medium supplemented with 0.75 mm H2O2 (D). Error bars represent the S.D. from three biological experiments. The P-values of the data were calculated by unpaired two-tailed Student's t test using GraphPad Prism 7. Asterisks denote significant difference (***, P < 0.001, two-tailed Student's t test) between two groups.
H2O2 decreases the DNA-binding ability of CmtR both in vitro and in M. bovis
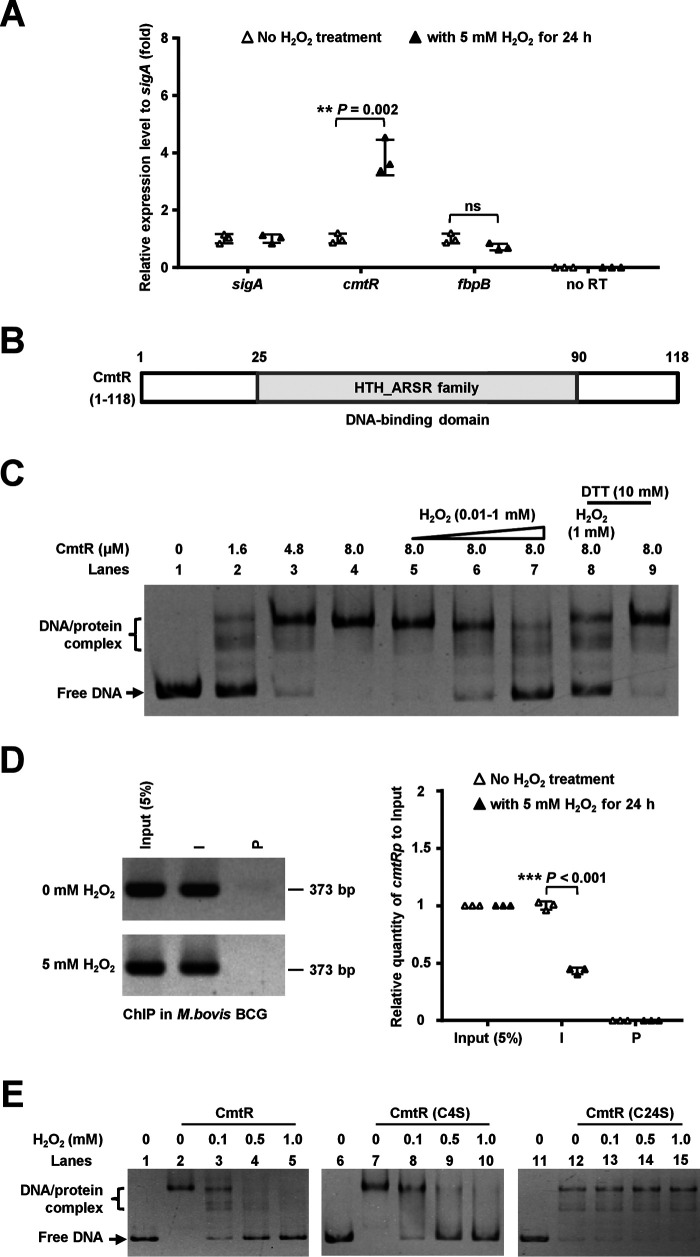
To elucidate the CmtR-triggered signaling pathway that regulates mycobacterial resistance to oxidative stress, we first determined whether the redox reagent, H2O2, induces cmtR expression in M. bovis BCG. To this end, we performed a qRT-PCR assay to evaluate the differential expression of M. bovis cmtR in the presence or absence of H2O2. As shown in Fig. 2A, cmtR expression was ∼3.8-fold up-regulated in the presence of 5 mm H2O2 compared with that in its absence. In contrast, the expression of the negative control gene fbpB remained unaffected under the same experimental conditions. This result indicates that H2O2 induces the expression of cmtR in the mycobacterial strain.
Figure 2.
Assays for studying the effects of H2O2 on the DNA-binding ability of CmtR. A, qRT-PCR assays for cmtR expression in M. bovis BCG strains under 5 mm H2O2 stress. B, domain assay for M. tuberculosis CmtR by searching the Conserved Domain Database (CDD database) in the NCBI website. C, EMSA for studying the effects of H2O2 and DTT on the DNA-binding activity of CmtR. The CmtR promoter DNA substrate was co-incubated with increasing concentrations of CmtR (1.6-8.0 μm) (lanes 2–4) in the presence of H2O2 (lanes 5–7 and lane 8) or DTT (lanes 8 and 9). D, ChIP assays for studying the effect of H2O2 on the intracellular DNA-binding activity of CmtR in M. bovis BCG. The Input (5%) indicated that the supernatant of disrupted cells was diluted to 5% and used as the PCR template. ChIP using preimmune (P) or immune (I) sera raised against CmtR. The expression levels were quantified using qPCR (right panel). The P-values of the data were calculated by two-tailed Student's t test using GraphPad Prism 7. Asterisks represent significant difference (**, P < 0.01; ***, P < 0.001; ns, not significant, two-tailed Student's t test) between two groups. E, assays for studying the effect of H2O2 on the DNA-binding activity of mutant CmtR-C24S proteins. The protein concentration was 3.2 μm, and the different concentrations of H2O2 are indicated at the top of the panels. The protein/DNA complexes and free DNA are indicated by arrows on the left side of the panels.
Next, we investigated the mechanism underlying the induction of cmtR expression by H2O2. CmtR contains a helix-turn-helix motif (Fig. 2B) and has been reported to specifically bind to the upstream fragment of the cmtR operon (36, 38). We confirmed the previous observation, as shown in the Fig. 2C When increasing quantities of CmtR (1.6–8.0 μm) were added to the reaction mixtures, the bands corresponding to CmtR and its promoter complex were observed to shift, and a corresponding increase was observed in the percentage of protein/DNA complexes (lanes 2 and 3). In contrast, CmtR could not bind with the fbpBp DNA substrate (Fig. S2A, lanes 6–8). Furthermore, ChIP assays were performed to investigate the specific binding of CmtR to its own promoter in M. bovis BCG. The cmtR promoter cmtRp could be specifically recovered using CmtR antibody (Fig. S2B). However, a negative control promoter (fbpBp) could not be recovered using CmtR antisera under similar conditions. The specificity of CmtR antibodies was confirmed using a cmtR deletion strain (Fig. S2B, right panel). These results indicate that cmtRp is the target promoter of CmtR in M. bovis BCG. We further assayed the effects of H2O2 on the DNA-binding ability of CmtR. As shown in Fig. 2C, a stepwise reduction in the concentration of the specific protein/DNA complex was observed upon the addition of 0.01–1 mm H2O2 into the reaction mixtures (lanes 5–7). Notably, this dissociation could be reversed with the addition of the reducing agent DTT (lane 8). No effect was observed when only 10 mm DTT was added into the reaction mixture (lane 9), which indicates that the ability of CmtR to bind to DNA was specifically inhibited in the presence of H2O2. This was confirmed in the quantitative ChIP assay. As shown in Fig. 2D, the ChIP and qPCR assays revealed that CmtR could precipitate ∼2.3-fold less promoter DNA of the cmtR operon in the presence of 5 mm H2O2 than in its absence. In contrast, no significant difference was observed when EthR was used as a control regulatory protein in the ChIP and qPCR assays under the same experimental conditions (Fig. S2C). Collectively, H2O2 inhibits the DNA-binding ability of CmtR both in vitro and in M. bovis BCG.
Cys-24 residue of CmtR plays a unique role in H2O2 resistance of M. bovis
Cysteine residues have been shown to be responsible for redox-sensing in multiple transcriptional regulators (13). To identify the cysteine residue responsible for sensing oxidative stress in CmtR, we performed multiple sequence alignment of CmtR isolated from several mycobacterial species. As shown in Fig. S1B, six cysteine residues (Cys-4, Cys-24, Cys-35, Cys-57, Cys-61, and Cys-102) were conserved in certain important mycobacterial species, including M. bovis BCG and M. tuberculosis H37Ra and H37Rv (Fig. S1B). Site-directed mutations were further introduced at the sites encoding the cysteine residues in cmtR, and the DNA-binding activity of these mutant proteins was assayed in the electrophoretic mobility shift assay (EMSA). As shown in Fig. S3A, all the mutant proteins could retain their DNA-binding potential except for CmtR-C24C, the activity of which reduced marginally, and CmtR-C4S, which appeared to bind marginally more tightly than the WT protein. We next examined the effects of these mutations on the DNA-binding ability of CmtR under H2O2 stress. Notably, apart from that in CmtR-C24S, the mutations did not alter the sensitivity of CmtR to H2O2 (Fig. 2E and Fig. S3B). In contrast, treatment with H2O2 did not affect the DNA-binding activity of CmtR-Cys24S (Fig. 2E, lanes 11–15), which indicates that its potential to respond to the oxidative signal was not comparable with that of the WT protein. These results imply that the Cys-24 residue plays a unique role in responding to the H2O2 signal.
We further investigated the significance of the CmtR Cys24 residue in mycobacterial growth under H2O2 stress. As shown in Fig. S4B, the bacterial counts of both the cmtR-deleted strain and its cmtR-Cys24S complementation strain were significantly lower than that of the WT strain under 0.75 mm H2O2 stress for 4 and 6 days. In contrast, the WT cmtR gene could exactly complement the sensitive phenotype of the cmtR-deleted strain under H2O2 stress, and no growth difference was observed compared with the WT strain (Fig. S4A). These results indicate that the Cys-24 residue of CmtR plays a unique role in mycobacterial resistance to H2O2.
CmtR triggers the expression of the esx-3 operon genes
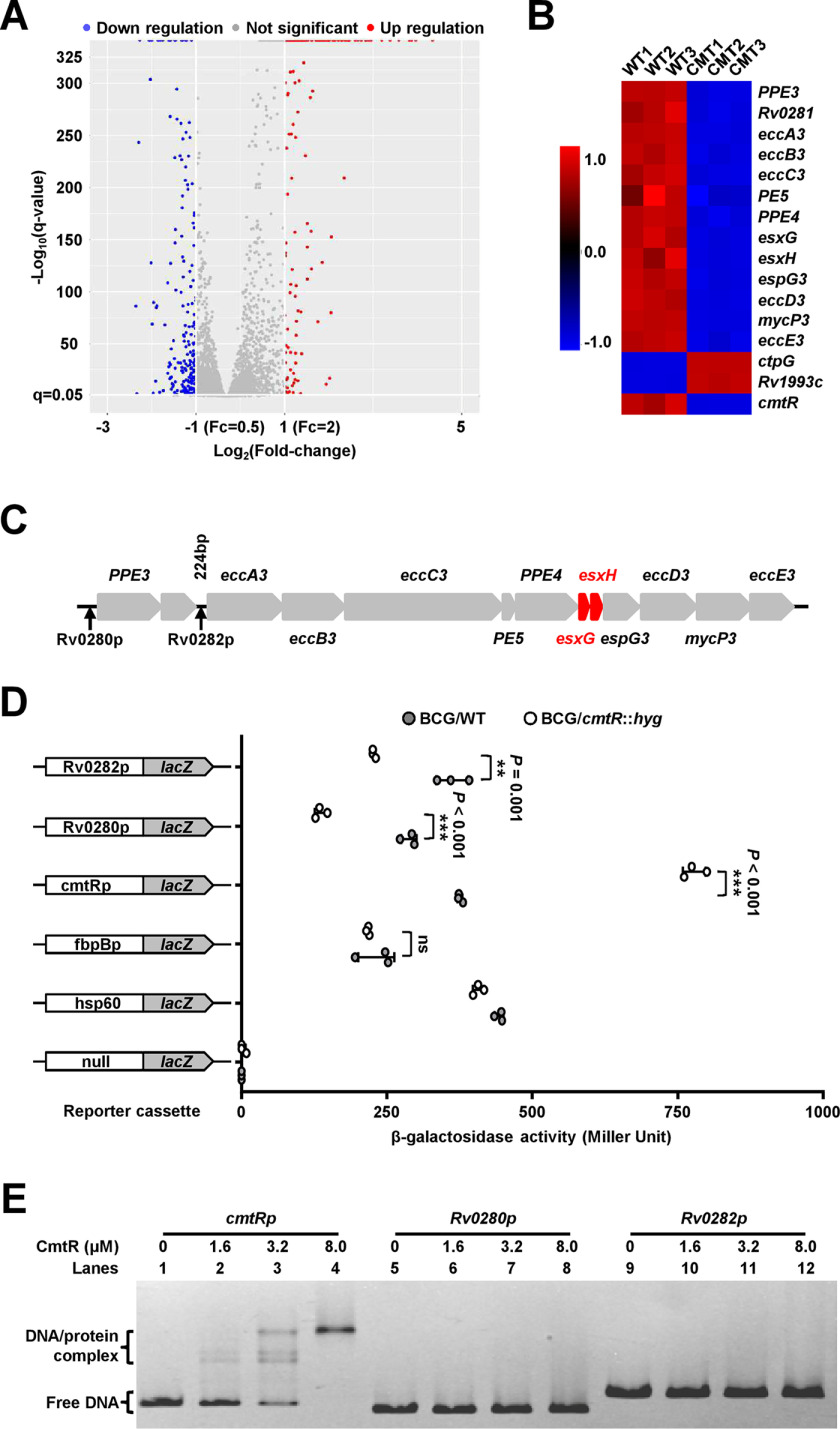
To further study the signaling pathway through which CmtR regulates mycobacterial resistance to H2O2, we performed RNA-Seq and transcriptomic assays to compare the differential gene expression between the WT and cmtR-deleted strains. As shown in Fig. 3A, the expression levels of 365 genes were observed to be affected by cmtR knockout. Notably, the esx-3 operon genes, which encode products essential for zinc and iron homeostasis, were integrally down-regulated in the cmtR-deleted strain compared with that in the WT strain (Fig. 3. B and C). In addition, the potential target regulatory genes of cmtR also include 12 inorganic ion transport- and metabolism-related genes, as shown in the evaluation of gene classification by the Cluster of Orthologous Groups analysis (Fig. S5). These data imply that CmtR may regulate metal ion homeostasis in mycobacterium.
Figure 3.
Regulatory effect of CmtR on the expression of the esx-3 operon. A, volcano plot of the difference in gene expression between WT and cmtR-deleted M. tuberculosis H37Ra strains determined using RNA-Seq assays. The x axis and y axis indicate the log2(fold-change) values and the −log10(q-value) values, respectively, of all genes. The Cuffdiff program was executed to perform differential expression tests using the edgeR package. The differential expression of a transcript is reported as significant if the test indicates that the false discovery rate–adjusted P-value for multiple-testing represents statistically significant values (q-value < 0.05). The significantly up-regulated and down-regulated genes are indicated by red and blue spots, respectively. Genes that did not undergo significant changes in expression are indicated by gray spots. B, heat map of the CmtR-regulated differential expression profile for the esx-3 operon and the cmtR operon. CMT1, CMT2, and CMT3 represent three biological replicates of differentially expressed genes in the cmtR knockout strain, respectively. WT1, WT2, and WT3 represent separately three biological replicates of the genes in the WT strain. C, the schematic of the esx-3 operon and its regulatory region. Two noncoding regions (rv0280p and rv0282p), which were potentially recognized by CmtR, are indicated by black arrows. D, assays for the promoter activities of rv0280p and rv0282p in the presence or absence of cmtR. β-gal activity was evaluated in both WT and cmtR-deleted strains of M. bovis BCG. Left column: schematic representation of recombinant strain generation using reporter plasmids. Null promoter-lacZ, hsp60-lacZ, and fbpBp-lacZ were used as controls. Error bars represent the S.D. from three biological experiments. The P-values of the data were calculated by two-tailed Student's t test using GraphPad Prism 7. Asterisks represent significant difference (**, P < 0.01; ***, P < 0.001; ns, not significant, two-tailed Student's t test) between two groups. E, EMSA for studying the DNA-binding activity of CmtR to cmtRp promoter DNA (lanes 1–4), rv0280p (lanes 5–8), and rv0282p (lanes 9–12). The protein/DNA complexes and free DNA are indicated by arrows on the left side of the panels.
The positive regulation of the esx-3 operon by CmtR in M. bovis BCG could be further confirmed by β-gal activity assays. As shown in Fig. 3D, hsp60p significantly promoted the expression of lacZ in different M. bovis BCG strains relative to the nonpromoter lacZ plasmid, which indicates that the reporting system functioned properly. cmtRp activated the expression of lacZ in the cmtR-deleted strain compared with that in the WT strain. In contrast, two esx-3p, rv0280p and rv0282p, significantly inhibited the expression of lacZ in the cmtR-deleted strain. However, no significant difference was observed in the expression of lacZ between the WT and cmtR-deleted strains when a negative control fbpBp was used as a promoter. These data indicate that although CmtR inhibits the expression of its own operon, it positively regulates the expression of the esx-3 operon.
The induction of the esx-3 operon by H2O2 depends on CmtR
A previous study revealed that the expression of the esx-3 operon was induced by H2O2 in M. tuberculosis H37Rv (39). Here, we observed CmtR can act as a redox sensor and positively regulate the esx-3 operon, which implies that H2O2 stimulates expression of the esx-3 operon most likely through the CmtR regulator. To confirm this, we compared the expression of the esx-3 operon genes in M. bovis BCG and cmtR-deleted strains in the presence or absence of oxidative stress. As shown in Fig. S6A, compared with that under no H2O2 stress, the expression of the esx-3 operon genes in M. bovis BCG, including Rv0281, esxG, and esxH, was significantly up-regulated in the presence of 5 mm H2O2. In contrast, no significant difference was observed in the expression of these genes in the BCG/cmtR::hyg strain under similar conditions (Fig. S6B). However, katG expression was always induced in response to 5 mm H2O2 treatment both in the WT and cmtR-deleted strains (Fig. S6). These results indicate that the high expression of the esx-3 operon (esxG and esxH) in response to H2O2 treatment depends on CmtR.
An EMSA was further performed to determine whether the purified CmtR protein can directly bind with the two esx-3 operon promoter DNA substrates, rv0280p and rv0282p. As shown in Fig. 3E, as increasing levels of CmtR (1.6–8 μm) were added into the reaction mixtures, a stepwise increase was clearly observed in the levels of shifted DNA when CmtR was co-incubated with the cmtRp (lanes 2–4), whereas the same was not observed with rv0280p (lanes 6–8) and rv0282p (lanes 10–12), which indicates that CmtR alone could not directly bind with the esx-3 operon promoter DNA. Collectively, these data indicate that CmtR could indirectly trigger the expression of the esx-3 operon in response to H2O2 stress.
CmtR physically interacts with Zur and neutralizes its inhibitory activity
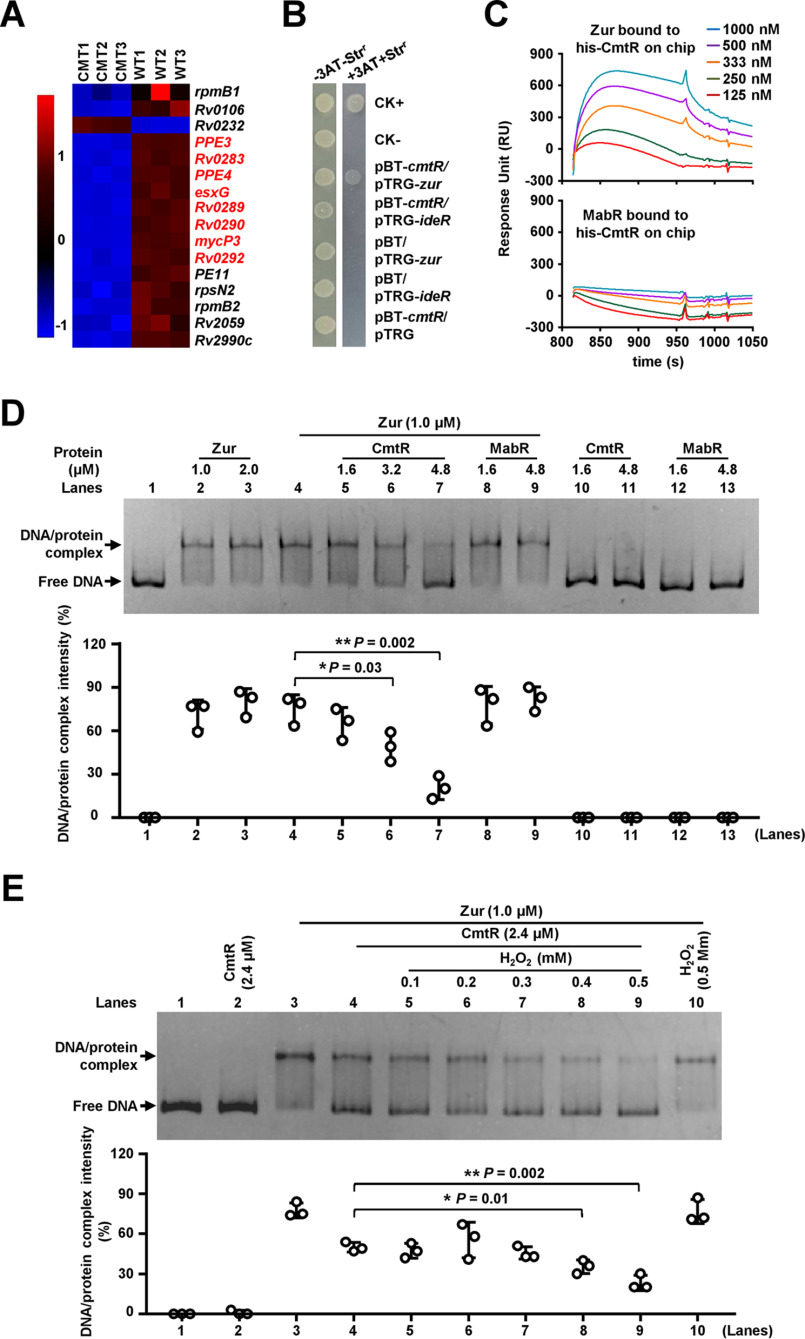
Next, we focused on transcriptomic data to evaluate the mechanism underlying the activation of the esx-3 operon by CmtR. Notably, except Rv0232, 16 genes inhibited by Zur (31) were significantly down-regulated in the cmtR-deleted strain (Fig. 4A). Of these, rpmB1(Rv0106), rpmB2(Rv2059), and the esx-3 operon genes have been implicated in zinc homeostasis. Such overlap between target genes that are regulated by two transcriptional factors is indicative of direct interaction between CmtR and Zur. To confirm this, we first studied the interaction in bacterial two-hybrid assays. IdeR has been reported to directly regulate the expression of the esx-3 operon (32), and was used as a negative control regulator. As shown in Fig. 4B, the co-transformant containing cmtR/zur was cultured successfully in the screening medium, which indicates that CmtR directly interacts with Zur. In contrast, the co-transformant containing cmtR/ideR did not grow under similar screening conditions. The controls containing cmtR, zur, or ideR alone did not grow under the same conditions. These results indicate that CmtR specifically interacts with Zur. Using purified transcriptional factor proteins for surface plasmon resonance (SPR) analysis, we confirmed the physical interaction between these two regulators. As shown in Fig. 4C, the corresponding response increased at increasing concentrations of Zur (125–1000 nm) over the His-tagged CmtR-immobilized nitrilotriacetic acid (NTA) chip. In contrast, no clear interaction was observed between CmtR and MabR under similar conditions. Therefore, Zur and CmtR interact physically.
Figure 4.
Assays for studying the interaction between CmtR and Zur. A, heat map of the CmtR-regulated differential expression profile of Zur-targeted genes. CMT1, CMT2, and CMT3 represent three biological replicates of the genes in the cmtR knockout strain. WT1, WT2, and WT3 represent three biological replicates of the genes in the WT strain. B, bacterial two-hybrid assays for the interaction between CmtR and Zur. E. coli reporter strains with various recombinant plasmids were spotted on the plate in the presence or absence of streptomycin (str) and 3-amino-1, 2, 4-triazole. ideR was used as a negative control. C, SPR assays for studying the specific interaction between CmtR and Zur. For studying the interaction between Zur and CmtR, His-tagged CmtR proteins were immobilized onto the NTA chips. Zur (top panel) protein and control MabR proteins (bottom panel) at different concentrations were passed over the chip. D, EMSA for studying the inhibitory effect of CmtR on the DNA-binding activity of Zur. The rv0280p DNA substrate was co-incubated with corresponding quantities of Zur (lanes 2–4), CmtR (lanes 10 and 11), or both (lanes 5–7). MabR was used as a negative control regulator. E, EMSA for studying the effect of H2O2 on the DNA-binding activity of Zur in the presence of CmtR. Error bars represent the S.D. from three independent experiments. The P-values of the data were calculated by unpaired two-tailed Student's t test using GraphPad Prism 7. Asterisks represent significant difference (*, P < 0.05; **, P < 0.01, two-tailed Student's t test) between two groups.
The direct interaction suggests that CmtR can affect the regulation of esx-3 operon expression by Zur. To confirm this, we first studied the effect of CmtR on the DNA-binding ability of Zur with the esx-3 operon promoter (rv0280p) by EMSA. As shown in Fig. 4D, with the addition of increasing levels (1.6–4.8 μm) of CmtR to the reaction mixture, a stepwise reduction was observed in the levels of shifted bonds corresponding to the specific Zur and rv0280p DNA complex (lanes 5–7). In contrast, no obvious effect was observed for MabR, an unrelated protein, under the same experimental conditions (lanes 8 and 9), which indicates that CmtR specifically inhibits the DNA-binding ability of Zur.
We further studied the integrated effect of H2O2 and CmtR on the DNA-binding activity of Zur using EMSA. As shown in Fig. 4E, at 1 μm, Zur could bind satisfactorily with rv0280p (lane 3). No bond shift was observed, as only 3.2 μm CmtR was added into the DNA-binding reaction mixture (lane 2). Upon the addition of 2.4 μm CmtR into the reaction mixture, a clear decrease was observed in the levels of shifted bonds (lanes 4). Notably, upon the addition of increasing concentrations of H2O2 (0.1–0.5 mm) to the reaction mixtures, the DNA substrates shifted by Zur were released continuously (lanes 5–9). These results indicate that H2O2 further enhances the CmtR-mediated inhibition of the DNA-binding activity of Zur.
Collectively, CmtR physically interacts with Zur and neutralizes its inhibitory activity. H2O2 can further enhance the CmtR-mediated inhibition of the DNA-binding activity of Zur.
Regulation of bacterial growth by CmtR depends on esxH expression
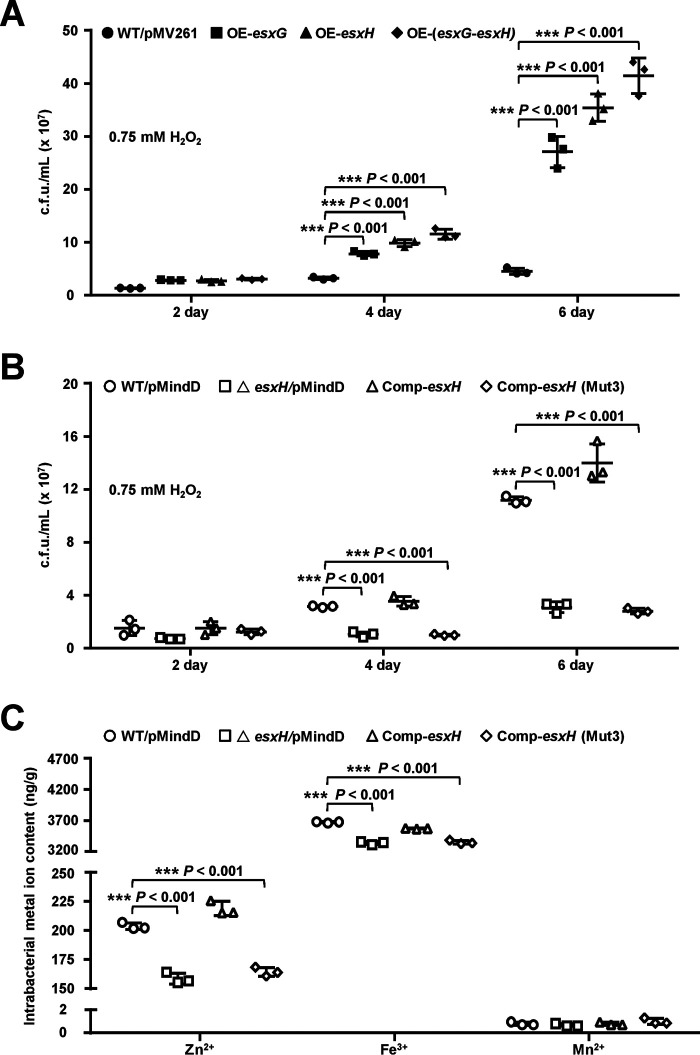
CmtR positively regulates the expression of the esx-3 operon genes, including esxG and esxH, by inhibiting the DNA-binding ability of Zur, and enhances mycobacterial H2O2-resistance, which implies that EsxG or EsxH contributes to mycobacterial resistance to oxidative stress. To confirm this, we studied the effects of esxG, esxH, and esxG-esxH overexpression on mycobacterial growth under H2O2 stress. As shown in Fig. 5A, when the plasmid pMV261 was used to overexpress esxG, esxH, or esxG-esxH in M. bovis BCG, the recombinant mycobacterial strains exhibited better growth than the WT strain when treated with 0.75 mm H2O2 for 4 and 6 days. No obvious growth difference was observed among these mycobacterial strains in the absence of H2O2 stress at the specified time points (Fig. S7A). These results indicate that EsxG or EsxH increases mycobacterial resistance to H2O2. We further constructed an esxH-deleted strain and constructed both WT esxH and its mutant gene esxH(Mut3), in which three zinc-binding amino acids (H14A, H70A, and H76A) were mutated, to form complementation M. bovis BCG strains. As shown in Fig. 5B, the bacterial counts of the esxH-deleted strain and the esxH3(Mut3) complementation strain were found to be significantly lower than those of the WT strains when treated with 0.75 mm H2O2 for 4 and 6 days. In contrast, no growth difference was observed among the strains in the absence of H2O2 at the specified time points (Fig. S7B). These results further demonstrate that EsxH contributes to the ability of mycobacteria to survive under oxidative stress.
Figure 5.
Assays for studying the effects of EsxH on intracellular Zn2+ accumulation in bacteria and mycobacterial growth under H2O2 stress. A, assays for studying the effects of esxG and esxH overexpression on the growth of M. bovis BCG strain under 0.75 mm H2O2 stress. WT/pMV261 represents the BCG/pMV261 strain; OE-esxG, OE-esxH, and OE-(esxG-esxH) represent the BCG/pMV261-esxG, BCG/pMV261-esxH, and BCG/pMV261-(esxG-esxH) strains, respectively. B, assays for studying the effects of esxH deletion on the growth of M. bovis BCG strain under 0.75 mm H2O2 stress. WT/pMindD represents the BCG/pMindD strain; △esxH/pMindD represents the BCG esxH::hyg/pMindD strain; Comp-esxH represents the BCG esxH::hyg/pMindD-esxH strain; Comp-esxH-Mut3 represents the BCG esxH::hyg/pMindD-esxH (Mut3) (Mut3: H14A, H70A, H76A) strain. C, assays for studying the effects of esxH on metal ion accumulation in bacterial cells under H2O2 stress. Mycobacterial strains were cultured till the A600 value reached 0.5 and were subsequently treated with 5 mm H2O2 for 24 h. The metal ion concentration within bacterial cells was measured by ICP-OES. Error bars represent the S.D. from three biological experiments. The P-values of the data were calculated by unpaired two-tailed Student's t test using GraphPad Prism 7. Asterisks represent significant difference (***, P < 0.001; two-tailed Student's t test) between two groups.
The EsxG-EsxH complex had been observed to possess a specific Zn2+-binding site formed from a cluster of histidine residues in EsxH (34), which implies that their expression levels have a potentially important effect on bacterial zinc homeostasis, and the regulation of mycobacterial growth potential under antioxidant stress by CmtR could be related to zinc homeostasis. To confirm this, we determined the zinc concentrations in several recombinant strains using inductively coupled plasma optical emission spectrometry (ICP-OES). As shown in Fig. 5C, consistent with the findings of the previous study, the intracellular Fe3+ contents in the esxH-deleted strain and the esxH(Mut3) complementation strain were significantly lower than that in the WT strain under 5 mm H2O2 stress. Notably, a similar result was obtained for the intracellular Zn2+ content (Fig. 5C). In contrast, no difference was observed in the intracellular Mn2+ content of these strains under the same experimental conditions. These results indicate that EsxH contributes to the antioxidant potential of mycobacteria and affects zinc homeostasis in mycobacterium.
CmtR regulates EsxH-dependent Zn2+ accumulation in M. bovis BCG
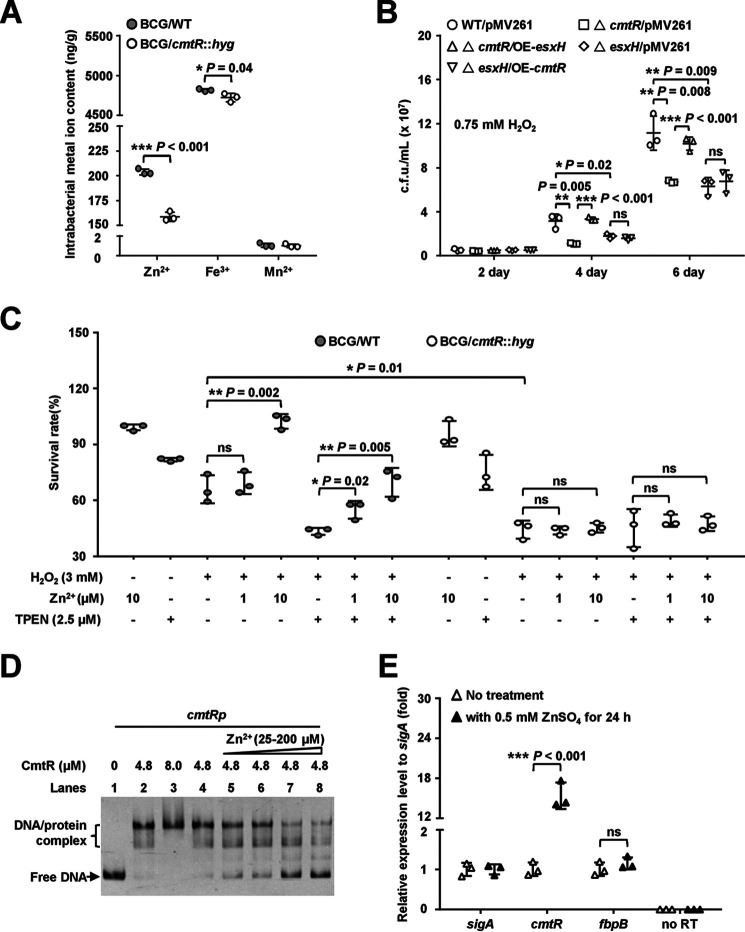
CmtR triggers the expression of the esx-3 operon genes, including esxH, the product of which affects zinc homeostasis in M. bovis, which implies that CmtR may regulate the intracellular Zn2+ content under oxidative stress. To confirm this, we assayed the Zn2+ content in the WT and cmtR-deleted strains. As shown in Fig. 6A, the levels of both intracellular Zn2+ and Fe3+ in the cmtR-deleted M. bovis strain were significantly lower than those in the WT strain under 5 mm H2O2 stress. In contrast, the Mn2+ content remained unaffected in the cmtR-deleted strain under the same experimental conditions. These data suggest that CmtR is essential for the intracellular accumulation of Zn2+ and Fe3+ under H2O2 stress.
Figure 6.
Assays for studying the effect of CmtR on intracellular metal ion accumulation in bacteria and mycobacterial growth under oxidative stress. A, ICP-OES assay for measuring the intracellular metal ion contents of WT and cmtR-deleted strains of M. bovis BCG under 5 mm H2O2 stress. B, assays for studying the effects of esxH-dependent regulation of cmtR on the growth of M. bovis BCG strain under 0.75 mm H2O2 stress. WT/pMV261 represents the BCG/pMV261 strain; △cmtR/pMV261 represents the BCG cmtR::hyg/pMV261 strain; △cmtR/OE-esxH represents the BCG cmtR::hyg/pMV261-esxH strain; △esxH/pMV261 represents the BCG esxH::hyg/pMV261 strain; △esxH/OE-cmtR represents the BCG esxH::hyg/pMV261-cmtR strain. C, assays for studying the essential role of CmtR in the enhancement of exogenous Zn2+ on antioxidant ability of M. bovis BCG strains. The cmtR-deleted and WT strains were cultured in Sauton's medium till the A600 value reached 0.5, and the culture was then supplemented with or without Zn2+, the Zn2+ chelator N, N, N′, N′-tetrakis (2-pyridylmethyl) ethylenediamine), and H2O2 for 24 h. D, EMSA for studying the effects of Zn2+ on the DNA-binding activity of CmtR. The cmtRp DNA substrate was co-incubated with CmtR in the absence (lanes 1–4) or presence of Zn2+ (25-200 μm) (lanes 5–8). E, qRT-PCR assays for studying the induction of cmtR expression in M. bovis BCG strain by Zn2+. Error bars represent the S.D. from three biological experiments. The P-values of the data were calculated by unpaired two-tailed Student's t test using GraphPad Prism 7. Asterisks represent significant difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant; two-tailed Student's t test) between two groups.
Further, we confirmed that the effect of CmtR on mycobacterial H2O2 resistance depends on the expression of esxH and the intracellular accumulation of Zn2+. We first determined if CmtR-mediated regulation of mycobacterial H2O2 resistance depends on the expression of EsxH. As shown in Fig. 6B, compared with the WT strain, the growth of the cmtR-deleted or esxH-deleted strains was significantly inhibited under 0.75 mm H2O2 stress for 4 and 6 days. When esxH was overexpressed in the cmtR-deleted strain, the recombinant strain exhibited better growth than the cmtR-deleted strain; however, when cmtR was overexpressed in the esxH-deleted strain, no obvious growth difference was observed under similar stress conditions. In contrast, no growth difference was observed among these strains in the absence of H2O2 stress (Fig. S7C). In addition, the survival rates of the cmtR-deleted or esxH-deleted strains were significantly lower than that of the WT strain, as shown in Fig. S8. When esxH was overexpressed in the cmtR-deleted strain, the survival rate of the recombinant strain was higher than that of the cmtR-deleted strain; however, no obvious difference was observed when cmtR was overexpressed in the esxH-deleted strain. These results indicate that the effect of CmtR on mycobacterial H2O2 resistance depends on EsxH.
Next, we evaluated whether the intracellular accumulation of Zn2+ contributes to bacterial survival under H2O2 stress. As shown in Fig. 6C, the WT strain exhibited better growth than the cmtR-deleted strain in the presence of 3 mm H2O2. Under 3 mm H2O2 stress, the addition of exogenous Zn2+ led to the rescue of the growth of the WT strain, whereas that of the cmtR-deleted strain was not rescued. Furthermore, the inhibitory effects of TPEN and H2O2 could be partly neutralized by increasing the concentration of exogenous Zn2+ in a dose-dependent manner in the WT strain culture, whereas the same could not be achieved in the cmtR-deleted strain culture. These data indicate that the intracellular accumulation of Zn2+ contributes to the potential of growth under H2O2 stress in mycobacteria.
Zn2+ inhibits the DNA-binding activity of CmtR both in vitro and in M. bovis
It has been shown previously that CmtR binds Zn2+ in vitro (37), which implies that Zn2+ may affect the DNA-binding activity of CmtR. An EMSA was conducted to validate this possibility. As shown in Fig. 6D, CmtR (4.8–8 μm) could bind satisfactorily with the cmtRp DNA substrate (lanes 2 and 3). When increasing quantities of Zn2+ (25–200 μm) were added to the reaction mixtures (lanes 5–8), the levels of shifted DNA substrates decreased correspondingly, which indicates that Zn2+ inhibited the DNA-binding activity of CmtR. The findings from the qRT-PCR assay further confirmed the effect of Zn2+ on the expression of cmtR in M. bovis BCG strain. As shown in Fig. 6E, cmtR expression was up-regulated by ∼15-fold in the presence of 0.5 mm Zn2+ compared with that under no treatment. In contrast, the expression of the control gene fbpB remained unaffected under the same experimental conditions. These results indicate that Zn2+ inhibits the DNA-binding activity of CmtR and induces the expression of cmtR in mycobacterial strains.
CmtR enhances M. bovis survival within the host
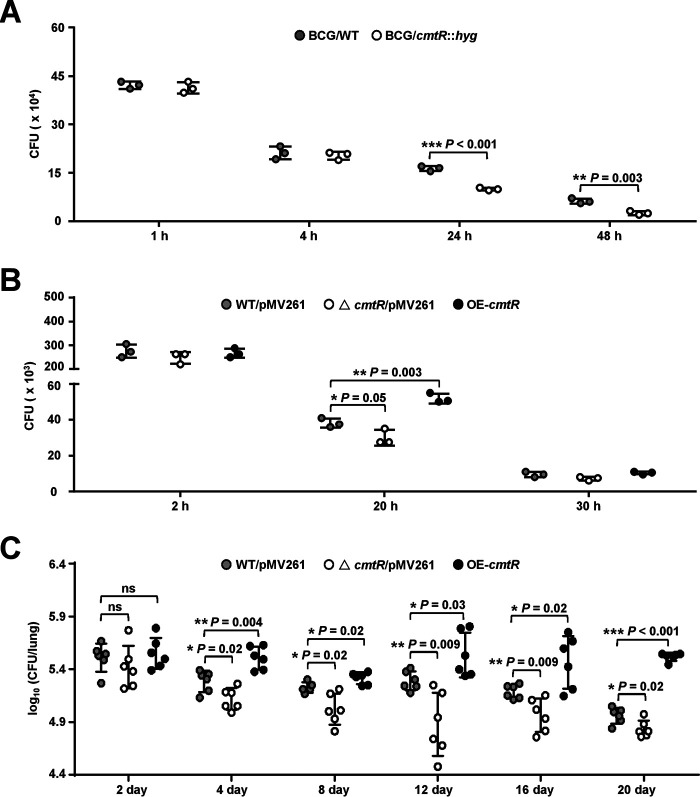
CmtR enhances the ability of M. bovis to proliferate under oxidative stress and promotes the expression of esxH, which implies that this regulator could contribute to bacterial survival in the host. To confirm this, we utilized two cell models to evaluate the effects of CmtR on the survival of M. bovis. First, RAW264.7 macrophages were infected with WT and cmtR-deleted strains. The results revealed that both strains invaded macrophages comparably at 1 h post infection (hpi). However, the intracellular survival efficiency of the cmtR-deleted strain in macrophages was significantly lower than that of the WT strain at 24 and 48 hpi (Fig. 7A). Similarly, the survival efficiency of the cmtR-deleted strain was significantly lower than that of the WT strain in bone marrow–derived macrophages (BMDMs) at 20 and 30 hpi, whereas the survival efficiency of the cmtR-overexpressing strain was observably higher than that of the WT strain (Fig. 7B). These results strongly suggest that CmtR enhances mycobacterial survival in macrophages.
Figure 7.
Assays for studying the effect of CmtR on intracellular survival of mycobacteria in macrophages and in mice. Cells were infected with mycobacterial strains at a multiplicity of infection of 10 and washed three times at 4 h post infection to remove extracellular bacteria. Thereafter, the cells were re-incubated in a medium supplemented with penicillin/streptomycin and lysed using 0.025% SDS at the indicated post-infection time points. Serial dilutions of the supernatant were then plated on 7H10 agar supplemented with 10% oleic acid–albumin–dextrose–catalase, and the number of cfu (CFU) was counted 15–21 days later. A, RAW264.7 cells were infected with BCG/WT and BCG/cmtR::hyg strains separately. B, BMDMs were infected with WT/pMV261 (BCG/pMV261), △cmtR/pMV261 (BCG cmtR::hyg/pMV261), and OE-cmtR (BCG/pMV261-cmtR) strains, respectively. C, female SPF C57BL/6 mice (n = 6 mice per group) were infected intratracheally with 1 × 106 of WT/pMV261, △cmtR/pMV261, and OE-cmtR strains for 0–20 days, and the bacterial loads in lung tissue homogenates of mice were determined. Error bars represent the S.D. from three biological experiments. The P-values of the data were calculated by unpaired two-tailed Student's t test using GraphPad Prism 7. Asterisks represent significant difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant; two-tailed Student's t test) between two groups.
Next, we determined the bacterial loads in the lungs of C57BL/6 mice infected with the WT, cmtR-deleted, and cmtR-overexpressing M. bovis strains. As shown in Fig. 7C, the bacterial load in the lung tissues of mice infected with all three strains were similar at 2 days post infection (dpi); however, the bacterial load in the lungs of mice infected with the cmtR-overexpressing strain increased significantly compared with that of mice infected with the WT strain from 4 to 20 dpi. In contrast, compared with the mice infected with the WT strain, those infected with the cmtR-deleted strain exhibited an obvious decrease in bacterial load since 4 dpi, which indicates that CmtR enhances the M. bovis load in the lungs of mice. Collectively, our data suggest that CmtR enhances mycobacterial survival in macrophages in vitro as well as in mice.
Discussion
Mtb is considered one of the most persistent intracellular pathogens and has developed unique mechanisms to adapt to host environments during infection. However, upon sensing oxidative stress, the molecular mechanism by which mycobacteria adaptively promote antioxidant regulation, and the associated signaling pathways, remains largely unclear. In this study, using M. bovis BCG as a model, we first characterized CmtR as a novel redox sensor containing an essential cysteine residue for sensing oxidative stress signals. We further observed that CmtR can physically interact with Zur and is associated with the expression regulation of the esx-3 operon and maintenance of Zn2+ homeostasis in bacterial cells. Lastly, we provided evidence to demonstrate that CmtR affects mycobacterial interaction with host cells and contributes to bacterial survival under stress and during infection. Our findings revealed the existence of a novel antioxidant defense pathway and regulatory mechanism that mycobacteria respond to and adapt to in the host environment.
To date, only a number of redox-sensing regulators have been characterized in mycobacterial species, including MosR (18) and OxyS (19). Notably, these regulators function as redox-dependent transcription factors that can directly recognize the upstream regulatory sequence of the redox gene cluster. For example, MosR senses and responds to oxidative stress and regulates the expression of a putative exported oxidoreductase (18), whereas OxyS regulates the expression of katG (19). This represents the model for the regulation of antioxidant defense in bacteria by a redox sensor. In the present study, we successfully characterized CmtR as a novel redox sensor, which contains an essential cysteine residue for sensing redox signals. Moreover, we found that CmtR does not directly bind with the upstream regulatory sequence of the esx-3 operon; instead, it physically interacts with Zur, a zinc uptake repressor of the operon (31). This eventually leads to the de-repression of Zur regulation and up-regulates the expression of the esx-3 operon, which enhances Zn2+ accumulation in bacterial cells and triggers ROS detoxication. Therefore, in contrast to the mechanism followed by redox sensors reported earlier, the regulation of antioxidant defense by CmtR represents a novel model for antioxidant defense in mycobacteria.
Metal ions are strongly associated with bacterial survival under oxidative stress. Fe2+ ions are widely associated with such conditions, and they frequently cause oxidative stress via the Fenton reaction in the presence of oxygen. Zn2+ is an essential trace element localized to the active center or in a structurally important site in several bacterial proteins (23, 40). In particular, Zn2+ is also a cofactor for more than 300 enzymes, including several oxidoreductases, such as superoxide dismutase and alcohol dehydrogenase (40). Therefore, Zn2+ homeostasis is related to bacterial adaptation to oxidative stress. Meanwhile, certain intracellular pathogens must adapt to host-imposed zinc toxicity (41, 42). Notably, in M. tuberculosis, ESX-3, one of the type-VII secretion systems, was recently found to have a zinc transportation function. Interestingly, the expression of the esx-3 operon is regulated by Zur to maintain zinc homeostasis in M. tuberculosis (31, 33). In the present study, we found that CmtR can physically interact with Zur and inhibit the Zur-mediated repression of esx-3 operon. Deletion of cmtR significantly inhibited the esx-3 operon expression and bacterial growth under oxidative stress. This links CmtR to the regulation of the operon and bacterial survival under both oxidative stress and during infection. Consistently, we provided data to confirm that the overexpression of the special transport system product, EsxH, could improve Zn2+ concentration in bacterial cells and the corresponding potential for bacterial growth under oxidative stress. This is a novel regulatory pathway for mycobacterial adaptation to a stressful environment.
An interesting finding from the present study is that by influencing the expression of a type-VII secretion system, the function of CmtR, a redox-sensing regulator, could be linked to the interaction between mycobacteria and the host. The genome of M. tuberculosis encodes five ESX type-VII secretion systems, which can export a variety of proteins linked to tuberculosis pathogenesis (25, 26). ESX-3 is conserved in several mycobacterial species and can function as zinc transporter. Meanwhile, one of the ESX components, EsxH, can target the host endosomal sorting complex to impair phagosome maturation and the recognition of Mtb-infected cells (27, 28). In addition, the expression and secretion of the EsxG-EsxH heterodimer complex have been found to significantly affect M. tuberculosis–macrophage interaction and bacterial survival in host cells (29, 30). Consistently, in the present study, we observed that CmtR promotes mycobacterial virulence. Mice infected with the cmtR-overexpressing M. bovis BCG strain exhibited a higher bacterial load in the lungs than those infected with the WT strain. These are consistent with the finding that CmtR positively affected the expression of the esx-3 operon, as well as with findings from previous reports.
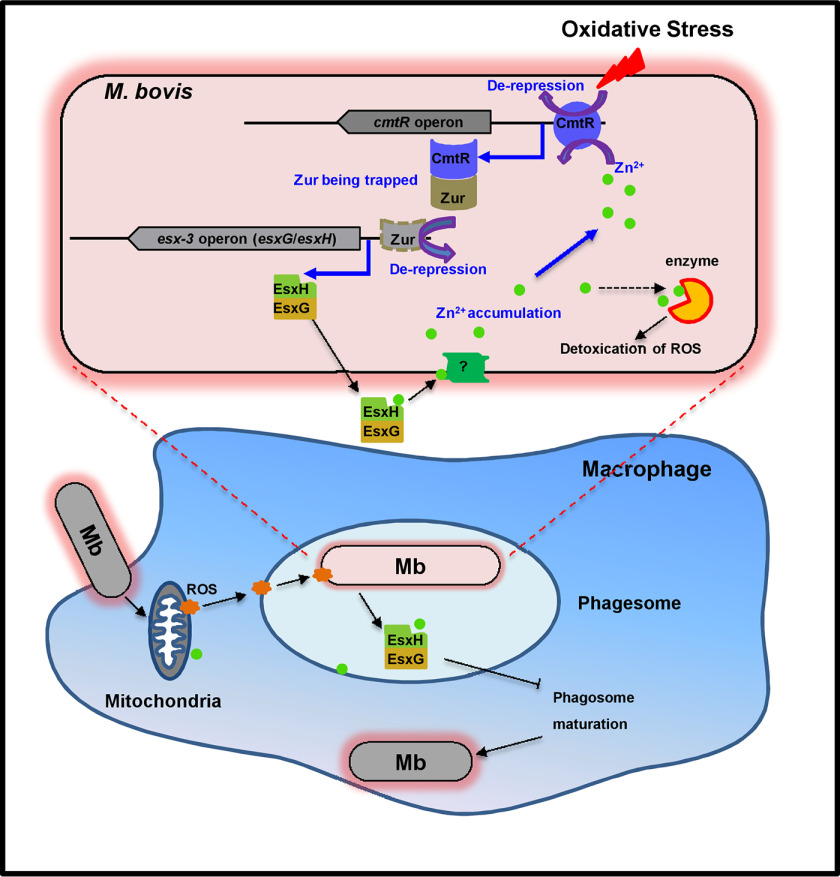
In summary, we characterized CmtR as a novel redox sensor in M. bovis BCG and showed that the regulation antioxidant defense by CmtR is a part of a novel signaling pathway. Our data support a model in which the expression of the redox sensor CmtR is induced in response to oxidative stress, following which CmtR targets the repressor Zur and de-represses the esx-3 operon (Fig. 8). On one hand, EsxH and EsxG transport Zn2+ into bacterial cells to promote ROS detoxication. On the other hand, both EsxH and EsxG impair phagosome maturation and the recognition of Mtb-infected cells. Both pathways aid mycobacterial survival during infection. Our findings reveal a novel signaling pathway and the molecular mechanism underlying antioxidant defense in M. bovis and provide insights into stress-induced mycobacterial adaptation to the host environment.
Figure 8.
A model depicting that the CmtR-ESX3-Zn2+ signaling pathway triggered by oxidative stress contributes to the survival of M. bovis in the host. Upon sensing an oxidative stress signal, CmtR expression is induced significantly, and it targets the negative regulator Zur to de-repress its inhibition on the expression of esxG and esxH, which triggers the accumulation of Zn2+ in the cytoplasm of M. bovis. Zn2+ subsequently activates antioxidant enzymes for combating oxidative stress in the host cell. Conversely, Zn2+ can de-repress the self-inhibition of cmtR, and further trigger the antioxidant regulatory signaling pathway.
Experimental procedures
Plasmids, enzymes, and reagents
The pET28a and pET28a-SUMO plasmids obtained from Novagen were used for overexpressing the mycobacterial protein in E. coli BL21 (DE3) strain (Novagen, Darmstadt, Germany). The pBT and pTRG vectors and the E. coli XR strains used for the bacterial two-hybrid assay were purchased from Stratagene (La Jolla, CA, USA). The primers for PCR were synthesized by Tsingke (China); these have been listed in Table S1. DNA polymerase, restriction enzymes, T4 ligase, dNTPs, and the antibiotics used were obtained from TaKaRa Biotech (Shiga, Japan). Ni2+-NTA–agarose was purchased from Qiagen (Hilden, Germany). The 7H9/7H10 medium and oleic acid–albumin–dextrose–catalase enrichment, which were used for mycobacterial growth, were purchased from BD Biosciences. Antisera were obtained from the Wuhan Animal Center of the Chinese Academy of Sciences (Wuhan, China).
Expression and purification of recombinant proteins
Genes were amplified using PCR with specific primer pairs (5′-CCACGAATTCGTATGCTGACGTGTGAGATGCG-3′ and 5′-CCGATCTAGATCTCAGCTACCTGTCATCTCGA-3′ for cmtR; 5′-AAAAATGGATCCATGAGTGCAGCCGGTGTC-3′ and 5′-CCAAAAGCTTTTAGCTC CGGCAGTCTGA-3′ for zur; 5′-ATTAGGATCCGTGAACGACAATCAGTTGGC-3′ and 5′-ATTAAAGCTTTCAGTGCGGCGTCGGATAGT-3′ for mabR). Several mutant genes were obtained through site-directed mutagenesis by overlapping extension PCR. The amplified DNA fragments were digested using the corresponding restriction endonucleases and cloned into the pET-28a or pET28a-SUMO expression vectors to produce recombinant plasmids (Table S2). The expression strains of E. coli BL21 (DE3) containing the recombinant plasmids were cultured, and the recombinant proteins were purified, as described in a previous study (43). The SUMO fusion protein was cleaved using the SUMO protease ULP1 to remove the tag (44). The eluate was dialyzed using dialysis buffer (20 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1 mm l-Arg, 10% glycerin) for 4 h at 4°C and stored at −80°C. Protein concentration was determined using the Coomassie Brilliant Blue assay.
EMSA
The DNA substrates for EMSA were amplified from M. tuberculosis H37Rv genomic DNA by PCR. The sequences of oligonucleotides fragments are listed in Table S1. The DNA-binding ability of proteins was evaluated using modified EMSA, as described previously (43). Briefly, the reaction mixtures (20 μl) for measuring mobility shift contained DNA fragments, Zur/CmtR/MabR at various concentrations, and buffer (20 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1 mm l-Arg, 10% glycerin) in the presence or absence of H2O2 at different concentrations. First, the proteins and H2O2 (at the indicated concentration) were co-incubated for 10 min on ice in dark. Next, the DNA substrates were added to the reaction mixtures and incubated for 20 min on ice. Lastly, the mixtures were electrophoresed in a 5% native polyacrylamide gel containing 1× Tris-glycine buffer at 150 V. Images were recorded using an FLA-5100 Fluorescent Image Analyzer (FUJIFILM, Japan).
Construction of recombinant mycobacterial strains
esxG, esxH, and esxH (H14A, H70A, H76A) genes were amplified by PCR using specific primer pairs (Table S1), and the amplicons were digested by the corresponding restriction endonucleases. The digested esxG, esxH, esxG-esxHI, and cmtR fragments, along with the corresponding mutant genes (mentioned above), were separately cloned into pMV261 overexpression vectors (45) or a pMindD vector (46) and transformed into M. bovis BCG WT and knockout strains to generate overexpression and complementary strains, respectively, in which the expression of the target genes was regulated using anhydrotetracycline hydrochloride. cmtR or esxH knockout was performed in M. bovis BCG or M. tuberculosis H37Ra strains, as described previously (47).
ChIP assay
The ChIP assay was performed as described previously with certain modifications (43). WT and cmtR-deleted M. bovis BCG strains were cultured until the A600 value reached 0.6 in 100 ml of 7H9 medium, after which the WT strains were separately supplemented with 0 or 5 mm H2O2 and cultured for 24 h. The bacterial cells were fixed with 1% formaldehyde, and the reactions were terminated using 0.125 M glycine. Next, the crosslinked cells were harvested and resuspended in 1 ml of TBST (TBS, 0.2% Triton X-100, 0.05% Tween 20). The sample was sonicated on ice, and the average DNA fragment size was determined to be 0.5–1.0 kb. The supernatant was collected from centrifuged samples. The special antibodies or pre-immune sera were added into 100 μl of the sample extracts under rotation for 3 h at 4°C. The complexes were immunoprecipitated by treating with 50 μl of 50% protein A-agarose for 1 h at 4°C. The immune complexes were recovered by centrifugation and resuspended in 50 μl TE (20 mm Tris-HCl, pH 7.8, 10 mm EDTA, 0.5% SDS). Next, the crosslinking was reversed by treatment for 6 h at 65°C. The DNA samples from the input and ChIP assay were purified and analyzed by PCR. The primer sequences are listed in Table S1. The protocol included one denaturation step of 5 min at 95°C, followed by 25 cycles of 20 s at 95°C, 20 s at 60°C, and 30 s at 72°C. The PCR products were separated by electrophoresis in 1.5% agarose gel containing 1 × Tris acetate-EDTA buffer at 100 V for 30 min.
Quantitative real-time PCR assays
mRNA extraction from the WT and cmtR-deleted M. bovis BCG strains and real-time PCR analysis were performed subsequently, as described earlier (19).
Quantitative ChIP assay
Quantitative ChIP assay is a quantitative PCR (qPCR) approach used for ChIP. The purified DNA samples of the input and ChIP assay were analyzed by qPCR (19).
Transcriptomic analysis
M. tuberculosis H37Ra strains (H37Ra/WT, H37Ra/cmtR::hyg) were cultured in 7H9 medium and subjected to shaking at 160 rpm at 37°C. The cells were cultured until the mid-logarithmic phase and harvested from each sample (each strain in three biological replicates). Subsequent transcriptomic analysis was performed, as described previously (47). In brief, total RNA was isolated using the RNeasy mini kit (Qiagen, Germany). Strand-specific libraries were prepared using the TruSeq® Stranded Total RNA Sample Preparation Kit (Illumina, USA) according to the manufacturer's instructions. Library construction and sequencing were performed at Shanghai Biotechnology Corporation.
The volcano plot diagrams were constructed using the −log10(q-value) and log2(fold-change) values of the genes between the WT and cmtR-deleted M. tuberculosis H37Ra strains using the ggplot2 package. Briefly, the cuffdiff program (48) was performed to conduct differential expression tests between the WT and cmtR knockout samples using the edgeR package (49). A transcript will be reported as differential expression significant if the test gives that the false discovery rate–adjusted P-value after Benjamini-Hochberg (50) correction for multiple-testing represents statistical significant (q-value < 0.05) (48, 51). The changes in gene expression are indicated on the x axis, and the q-values are indicated on the y axis. The red and blue spots represent the up-regulated and down-regulated genes, respectively, whereas the gray spots represent genes with insignificant changes in expression (Fig. 3A).
The heat map was constructed using the HemI (Heatmap Illustrator, version 1.0) software. Briefly, the fragments per kilobase of exon per million fragments mapped values of target genes in each sample were normalized and imported to the HemI software to construct the heat map diagram. Red represents high expression, whereas blue represents low expression of the target genes in different samples. The color scale beside the heat map indicates the color threshold.
Assay for β-gal activity
An assay for measuring β-gal activity was performed using the WT and cmtR-deleted M. bovis BCG strains by constructing operon-lacZ fusions based on the expression vector pMV261 (52). The target and control promoters were amplified by PCR using the respective primers, which are listed in Table S1, after which the amplicons were digested using the corresponding restriction endonucleases and cloned into the pMV261 backbone. The reporter gene lacZ (Table S1) was cloned downstream of the promoters. The plasmids were separately transformed into the cmtR-deleted and WT M. bovis BCG strains to obtain the corresponding recombinant reporter strains. The recombinant strains were cultured until the mid-logarithmic phase in 7H9 medium at 37°C. The bacterial cells were harvested and washed using PBS. The levels of galactosidase were measured as described previously (47).
Bacterial two-hybrid assay
The BacterioMatch II Two-Hybrid System (Stratagene) was used to detect interactions between CmtR and Zur or CmtR and IdeR, as described previously (53). The positive co-transformants were selected on the selective screening medium plate containing 5 mm 3-amino-1,2,4-triazole (Stratagene), 8 g/ml streptomycin, 15 g/ml tetracycline, 34 g/ml chloramphenicol, and 50 g/ml kanamycin (Kan). The co-transformants containing pBT-LGF2 and pTRG-Gal11p (Stratagene) were used as the positive controls, while those containing the empty vectors pBT and pTRG were used as the negative controls.
SPR analysis
The interaction between CmtR and Zur was analyzed using a Biacore 3000 instrument (GE Healthcare) according to previously published procedures (53). The His-tagged CmtR proteins (200 nm) were immobilized onto NTA chips, and 125–1000 nm Zur proteins were passed over the NTA chips at a flow rate of 10 ml/min at 25°C. For negative controls, Zur was substituted with MabR (125–1000 nm) under the same experimental conditions. The proteins were diluted using running buffer (10 mm HEPES, pH 7.4, 150 mm NaCl, 50 mm EDTA, and 0.005% Biacore surfactant P20) to the indicated concentration. Each analysis was performed in triplicate. Several overlay plots were constructed to depict the interactions using BIAevaluation 3.1 software.
Evaluation of mycobacterial growth
The growth patterns of M. bovis BCG strains were evaluated using modified versions of procedures described earlier (47). The recombinant strains were cultured in 7H9 medium supplemented with 30 g/ml Kan or supplemented with 50 ng/ml anhydrotetracycline hydrochloride for strains containing pMindD plasmids, and the cultures were incubated under shaking conditions at 160 rpm at 37°C. When the culture reached the mid-logarithmic phase, each culture was diluted (4:100) in 50 ml of fresh 7H9 broth containing the corresponding antibiotics mentioned above, and the cultures were divided into 10 equal volumes. Six of these were divided into two groups and separately treated with 0/0.75 mm H2O2 under shaking conditions at 160 rpm at 37°C. Next, 0.75 mm H2O2 was added to the medium at 0, 2, and 4 days to ensure that the medium contained significant levels of H2O2 throughout the duration of the experiment. The sensitivity of the recombinant strains to H2O2 stress was determined every 2 days. Serial dilutions of the samples were plated on 7H10 agar, and the number of cfu formed after 15–21 days was counted. Different WT and knockout strains were used during the experiments, and the concrete strains were indicated in the corresponding figure legend.
Determination of metal ion content under H2O2 stress
Total zinc, iron, and manganese ion contents in the dried bacterial pellets were determined by ICP-OES (Varian, USA) according to a previously published procedure (54) with several modifications. Briefly, the cmtR and esxH recombinant strains were cultured in 300 ml of 7H9 medium under shaking conditions at 160 rpm at 37°C until the A600 value reached 0.5, after which the strains were treated with 5 mm H2O2 for 24 h. The harvested samples were washed twice in PBS containing 1 mm EDTA and 0.05% Tween 80, and were then washed only with PBS. The pellets were stored overnight at −80°C and dried using the Freeze Dryer machine (Thermo Fisher). The dried samples were weighed and acid-digested with HNO3 (trace metal grade) for 4 h at 80°C and overnight at 65°C. The digestion experiments were terminated by adding one-eighth volume of 30% (v/v) H2O2 at 1:10 dilution with water. The supernatants were filtered by passing through 0.22-μm filters (Corning Inc.). The metal content in the digested samples was measured by ICP-OES.
Assays for the survival of mycobacteria under H2O2 stress
Assays for the sensitivity of mycobacteria to H2O2 were conducted as described previously (55) with certain modifications. Briefly, the cmtR-deleted and WT M. bovis BCG strains were cultured in 100 ml of 2× Sauton's medium supplemented with 0.2% glycerol, 0.05% Tween 80, 30 g/ml Kan. When the A600 value reached 0.5, each culture was divided into 27 equal volumes, further divided into nine groups, and separately treated with the indicated concentrations of Zn2+, the Zn2+ chelator N, N, N′, N′-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN) (Sigma), and H2O2 for 24 h. The cells were diluted and plated on 7H10 agar plates, and the cfu formed after 15-21 days were counted. The survival percentages were calculated based on cfu (with treatment)/cfu (without treatment) for each strain.
Mice
WT female SPF C57BL/6 mice were purchased from Changsheng Bio (Liaoning, China). The mice weighed 16–18 g and were 6–8 weeks old and housed in a specific pathogen-free facility using standard humane animal husbandry protocols that were approved by the Research Ethics Committee of the College of Veterinary Medicine, Huazhong Agricultural University, Hubei, Wuhan, China (HZAUMO-2019-013).
Preparation of BMDMs and infection
BMDMs were obtained by flushing the tibia and femurs of mice, as described previously (56). The cells were cultured in DMEM/F12 (HyClone, USA) supplemented with 10% FBS (Gibco, USA), 1% nonessential amino acid (NEAA, Gibco, USA), 1% Na-Pyrurate (Gibco, USA), 10% penicillin-streptomycin (Gibco, USA), and 30% L929 cell supernatant (containing macrophage colony-stimulating factor) in 5% CO2 for 3 days at 37°C, after which the well-mixed medium and L929 cell supernatant were added to the cultures for another 2 days. Mature BMDMs (5 × 105/well) were seeded in microculture dishes (Thermo Fisher Scientific) overnight and were subsequently used for bacterial infection.
Intracellular survival assays
The BMDMs and RAW264.7 cells were seeded in 24-well plates and cultured overnight. The cells were then infected with M. bovis BCG strains at a multiplicity of infection of 10, as described previously (56). At 4 hpi, the macrophages were washed thrice with PBS to remove extracellular bacteria and were then added to the well-mixed medium. After incubation for 1–48 h for RAW264.7 cells and for 2–30 h for BMDMs, the cells were lysed using 0.025% SDS and diluted for plating on 7H10 agar plates for counting cfu 15–21 days later. Penicillin/streptomycin was added to the medium, except during infections.
Mouse infection
The female SPF C57BL/6 mice weighed 16–18 g and were 6–8 weeks old during the experimental period. M. bovis BCG strains were cultured till the A600 value reached 1.0, and were washed three times with PBS containing 0.05% Tween 80. Thirty-six mice were randomly divided into six groups (n = 6), and were intratracheally infected with 1 × 106 cfu of WT/pMV261, △ cmtR/pMV261, and OE-cmtR strains separately, and data were analyzed as described previously (57).
Statistical analysis
Statistical analyses of data were performed using unpaired two-tailed Student's t test with GraphPad Prism 7. Data are expressed in terms of mean ± S.D. Asterisks represent significant difference, *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ns, not significant (p ≥ 0.05).
Data availability
All data described are presented either within the article or in the supporting information.
Supplementary Material
This article contains supporting information.
Author contributions—X. L., L. C., J. L., J. H., W. L., and Z.-G. H. conceptualization; X. L., J. H., and Z.-G. H. data curation; X. L., L. C., J. L., J. H., W. L., and Z.-G. H. formal analysis; X. L., L. C., J. L., J. H., W. L., and Z.-G. H. investigation; X. L., J. H., and Z.-G. H. writing-original draft; J. L., J. H., W. L., and Z.-G. H. project administration; J. H. and Z.-G. H. resources; J. H. and Z.-G. H. supervision; J. H. and Z.-G. H. funding acquisition; J. H. and Z.-G. H. writing-review and editing.
Funding and additional information—This work was supported by National Key R&D Program of China 2017YFD0500300, National Natural Science Foundation of China Grants 31730005 and 31670075, and Ba-Gui Scholar Program of Guangxi (to Z. G. H).
Conflict of interests—The authors declare that they have no conflict of interest with the contents of this article.
- ROS
- reactive oxygen species
- Mtb
- Mycobacterium tuberculosis
- BCG
- Bacillus Calmette-Guérin
- SPR
- surface plasmon resonance
- NTA
- nitrilotriacetic acid
- ICP-OES
- inductively coupled plasma optical emission spectrometry
- hpi
- hours post infection
- BMDM
- bone marrow–derived macrophage
- q
- quantitative
- Kan
- kanamycin.
References
- 1. Nathan, C., and Shiloh, M. U. (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 97, 8841–8848 10.1073/pnas.97.16.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambeth, J. D. (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- 3. Bedard, K., and Krause, K. H. (2007) The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 87, 245–313 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- 4. Ehrt, S., and Schnappinger, D. (2009) Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell Microbiol. 11, 1170–1178 10.1111/j.1462-5822.2009.01335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imlay, J. A. (2003) Pathways of oxidative damage. Annu. Rev. Microbiol. 57, 395–418 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 6. Brennan, P. J., and Nikaido, H. (1995) The envelope of mycobacteria. Annu. Rev. Biochem. 64, 29–63 10.1146/annurev.bi.64.070195.000333 [DOI] [PubMed] [Google Scholar]
- 7. Buchmeier, N., and Fahey, R. C. (2006) The mshA gene encoding the glycosyltransferase of mycothiol biosynthesis is essential in Mycobacterium tuberculosis Erdman. FEMS Microbiol. Lett. 264, 74–79 10.1111/j.1574-6968.2006.00441.x [DOI] [PubMed] [Google Scholar]
- 8. Buchmeier, N. A., Newton, G. L., and Fahey, R. C. (2006) A mycothiol synthase mutant of Mycobacterium tuberculosis has an altered thiol-disulfide content and limited tolerance to stress. J. Bacteriol. 188, 6245–6252 10.1128/JB.00393-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ng, V. H., Cox, J. S., Sousa, A. O., MacMicking, J. D., and McKinney, J. D. (2004) Role of KatG catalase-peroxidase in mycobacterial pathogenesis: Countering the phagocyte oxidative burst. Mol. Microbiol. 52, 1291–1302 10.1111/j.1365-2958.2004.04078.x [DOI] [PubMed] [Google Scholar]
- 10. Piddington, D. L., Fang, F. C., Laessig, T., Cooper, A. M., Orme, I. M., and Buchmeier, N. A. (2001) Cu, Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 69, 4980–4987 10.1128/IAI.69.8.4980-4987.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bryk, R., Griffin, P., and Nathan, C. (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 10.1038/35025109 [DOI] [PubMed] [Google Scholar]
- 12. Bryk, R., Lima, C. D., Erdjument-Bromage, H., Tempst, P., and Nathan, C. (2002) Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295, 1073–1077 10.1126/science.1067798 [DOI] [PubMed] [Google Scholar]
- 13. Green, J., and Paget, M. S. (2004) Bacterial redox sensors. Nat. Rev. Microbiol. 2, 954–966 10.1038/nrmicro1022 [DOI] [PubMed] [Google Scholar]
- 14. Choi, H. J., Kim, S. J., Mukhopadhyay, P., Cho, S., Woo, J. R., Storz, G., and Ryu, S. E. (2001) Structural basis of the redox switch in the OxyR transcription factor. Cell 105, 103–113 10.1016/S0092-8674(01)00300-2 [DOI] [PubMed] [Google Scholar]
- 15. Kim, S. O., Merchant, K., Nudelman, R., Beyer, W. F., Keng, T., DeAngelo, J., Hausladen, A., and Stamler, J. S. (2002) OxyR: A molecular code for redox-related signaling. Cell 109, 383–396 10.1016/S0092-8674(02)00723-7 [DOI] [PubMed] [Google Scholar]
- 16. Zheng, M., Åslund, F., and Storz, G. (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1722 10.1126/science.279.5357.1718 [DOI] [PubMed] [Google Scholar]
- 17. Fuangthong, M., Atichartpongkul, S., Mongkolsuk, S., and Helmann, J. D. (2001) OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis. J. Bacteriol. 183, 4134–4414 10.1128/JB.183.14.4134-4141.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brugarolas, P., Movahedzadeh, F., Wang, Y., Zhang, N., Bartek, I. L., Gao, Y. N., Voskuil, M. I., Franzblau, S. G., and He, C. (2012) The oxidation-sensing regulator (MosR) is a new redox-dependent transcription factor in Mycobacterium tuberculosis. J. Biol. Chem. 287, 37703–37712 10.1074/jbc.M112.388611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li, Y., and He, Z. G. (2012) The mycobacterial LysR-type regulator OxyS responds to oxidative stress and negatively regulates expression of the catalase-peroxidase gene. PLoS One 7, e30186 10.1371/journal.pone.0030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganguly, T., Kajfasz, J. K., Miller, J. H., Rabinowitz, E., Galvão, L. C., Rosalen, P. L., Abranches, J., and Lemos, J. A. (2018) Disruption of a novel iron transport system reverses oxidative stress phenotypes of a dpr mutant strain of Streptococcus mutans. J. Bacteriol. 200, e00062–18 10.1128/JB.00062-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Si, M., Zhao, C., Burkinshaw, B., Zhang, B., Wei, D., Wang, Y., Dong, T. G., and Shen, X. (2017) Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. U. S. A. 114, E2233–E2242 10.1073/pnas.1614902114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grunenwald, C. M., Choby, J. E., Juttukonda, L. J., Beavers, W. N., Weiss, A., Torres, V. J., and Skaar, E. P. (2019) Manganese detoxification by MntE is critical for resistance to oxidative stress and virulence of Staphylococcus aureus. MBio 10, e02915–18 10.1128/mBio.02915-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ortiz de Orué Lucana, D., Wedderhoff, I., and Groves, M. R. (2012) ROS-mediated signalling in bacteria: Zinc-containing Cys-XX-Cys redox centres and iron-based oxidative stress. J. Signal. Transduct. 2012, 605905 10.1155/2012/605905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang, T., Si, M., Song, Y., Zhu, W., Gao, F., Wang, Y., Zhang, L., Zhang, W., Wei, G., Luo, Z. Q., and Shen, X. (2015) Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLoS Pathog. 11, e1005020 10.1371/journal.ppat.1005020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bitter, W., Houben, E. N., Bottai, D., Brodin, P., Brown, E. J., Cox, J. S., Derbyshire, K., Fortune, S. M., Gao, L. Y., Liu, J., van Pittius, N. C. G., Pym, A. S., Rubin, E. J., Sherman, D. R., Cole, S. T., et al. (2009) Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 5, e1000507 10.1371/journal.ppat.1000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stoop, E. J., Bitter, W., and van der Sar, A. M. (2012) Tubercle bacilli rely on a type VII army for pathogenicity. Trends Microbiol. 20, 477–484 10.1016/j.tim.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 27. Mehra, A., Zahra, A., Thompson, V., Sirisaengtaksin, N., Wells, A., Porto, M., Köster, S., Penberthy, K., Kubota, Y., Dricot, A., Rogan, D., Vidal, M., Hill, D. E., Bean, A. J., and Philips, J. A. (2013) Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 9, e1003734 10.1371/journal.ppat.1003734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Portal-Celhay, C., Tufariello, J. M., Srivastava, S., Zahra, A., Klevorn, T., Grace, P. S., Mehra, A., Park, H. S., Ernst, J. D., Jacobs, W. R., Jr., and Philips, J. A. (2016) Mycobacterium tuberculosis EsxH inhibits ESCRT-dependent CD4+ T-cell activation. Nat. Microbiol. 2, 1–9 10.1038/nmicrobiol.2016.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tufariello, J. M., Chapman, J. R., Kerantzas, C. A., Wong, K.-W., Vilchèze, C., Jones, C. M., Cole, L. E., Tinaztepe, E., Thompson, V., Fenyö, D., Niederweis, M., Ueberheide, B., Philips, J. A., and Jacobs, W. R. (2016) Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc. Natl. Acad. Sci. U. S. A. 113, E348–E357 10.1073/pnas.1523321113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim, Y. S., Yang, C. S., Nguyen, L. T., Kim, J. K., Jin, H. S., Choe, J. H., Kim, S. Y., Lee, H. M., Jung, M., Kim, J. M., Kim, M. H., Jo, E. K., and Jang, J. C. (2017) Mycobacterium abscessus ESX-3 plays an important role in host inflammatory and pathological responses during infection. Microbes Infect. 19, 5–17 10.1016/j.micinf.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 31. Maciag, A., Dainese, E., Rodriguez, G. M., Milano, A., Provvedi, R., Pasca, M. R., Smith, I., Palù, G., Riccardi, G., and Manganelli, R. (2007) Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J. Bacteriol. 189, 730–740 10.1128/JB.01190-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodriguez, G. M., Voskuil, M. I., Gold, B., Schoolnik, G. K., and Smith, I. (2002) ideR, an essential gene in Mycobacterium tuberculosis: Role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect. Immun. 70, 3371–3381 10.1128/IAI.70.7.3371-3381.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Serafini, A., Pisu, D., Palù, G., Rodriguez, G. M., and Manganelli, R. (2013) The ESX-3 secretion system is necessary for iron and zinc homeostasis in Mycobacterium tuberculosis. PLoS One 8, e78351 10.1371/journal.pone.0078351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ilghari, D., Lightbody, K. L., Veverka, V., Waters, L. C., Muskett, F. W., Renshaw, P. S., and Carr, M. D. (2011) Solution structure of the Mycobacterium tuberculosis EsxG·EsxH complex: Functional implications and comparisons with other M. tuberculosis Esx family complexes. J. Biol. Chem. 286, 29993–30002 10.1074/jbc.M111.248732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tinaztepe, E., Wei, J. R., Raynowska, J., Portal-Celhay, C., Thompson, V., and Philips, J. A. (2016) Role of metal-dependent regulation of ESX-3 secretion in intracellular survival of Mycobacterium tuberculosis. Infect. Immun. 84, 2255–2263 10.1128/IAI.00197-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cavet, J. S., Graham, A. I., Meng, W., and Robinson, N. J. (2003) A cadmium-lead-sensing arsr-smtb repressor with novel sensory sites complementary metal discrimination by NmtR and CmtR in a common cytosol. J. Biol. Chem. 278, 44560–44566 10.1074/jbc.M307877200 [DOI] [PubMed] [Google Scholar]
- 37. Wang, Y., Hemmingsen, L., and Giedroc, D. P. (2005) Structural and functional characterization of Mycobacterium tuberculosis CmtR, a PbII/CdII-sensing SmtB/ArsR metalloregulatory repressor. Biochemistry 44, 8976–8988 10.1021/bi050094v [DOI] [PubMed] [Google Scholar]
- 38. Chauhan, S., Kumar, A., Singhal, A., Tyagi, J. S., and Krishna Prasad, H. (2009) CmtR, a cadmium-sensing ArsR-SmtB repressor, cooperatively interacts with multiple operator sites to autorepress its transcription in Mycobacterium tuberculosis. FEBS J. 276, 3428–3439 10.1111/j.1742-4658.2009.07066.x [DOI] [PubMed] [Google Scholar]
- 39. Namouchi, A., Gómez-Muñoz, M., Frye, S. A., Moen, L. V., Rognes, T., Tønjum, T., and Balasingham, S. V. (2016) The Mycobacterium tuberculosis transcriptional landscape under genotoxic stress. BMC Genomics 17, 791 10.1186/s12864-016-3132-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hantke, K. (2001) Bacterial zinc transporters and regulators. BioMetals 14, 239–249 10.1023/A:1012984713391 [DOI] [PubMed] [Google Scholar]
- 41. Botella, H., Peyron, P., Levillain, F., Poincloux, R., Poquet, Y., Brandli, I., Wang, C., Tailleux, L., Tilleul, S., Charrière, G. M., Waddell, S. J., Foti, M., Lugo-Villarino, G., Gao, Q., Maridonneau-Parini, I., et al. (2011) Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10, 248–259 10.1016/j.chom.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ong, C. L. Y., Gillen, C. M., Barnett, T. C., Walker, M. J., and McEwan, A. G. (2014) An antimicrobial role for zinc in innate immune defense against group A streptococcus. J. Infect. Dis. 209, 1500–1508 10.1093/infdis/jiu053 [DOI] [PubMed] [Google Scholar]
- 43. Yang, M., Gao, C., Cui, T., An, J., and He, Z. G. (2012) A TetR-like regulator broadly affects the expressions of diverse genes in Mycobacterium smegmatis. Nucleic Acids Res. 40, 1009–1020 10.1093/nar/gkr830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuo, D., Nie, M., and Courey, A. J. (2014) SUMO as a solubility tag and in vivo cleavage of SUMO fusion proteins with Ulp1. Methods Mol. Biol. 1177, 71–80 10.1007/978-1-4939-1034-2_6 [DOI] [PubMed] [Google Scholar]
- 45. Snapper, S. B., Melton, R. E., Mustafa, S., Kieser, T., and Jacobs, W. R., Jr. (1990) Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4, 1911–1919 10.1111/j.1365-2958.1990.tb02040.x [DOI] [PubMed] [Google Scholar]
- 46. Blokpoel, M. C., Murphy, H. N., O'Toole, R., Wiles, S., Runn, E. S., Stewart, G. R., Young, D. B., and Robertson, B. D. (2005) Tetracycline-inducible gene regulation in mycobacteria. Nucleic Acids Res. 33, e22 10.1093/nar/gni023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li, W., Li, M., Hu, L., Zhu, J., Xie, Z., Chen, J., and He, Z. G. (2018) HpoR, a novel c-di-GMP effective transcription factor, links the second messenger's regulatory function to the mycobacterial antioxidant defense. Nucleic Acids Res. 46, 3595–3611 10.1093/nar/gky146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trapnell, C., Roberts, A., Goff, L., Pertea, G., Kim, D., Kelley, D. R., Pimentel, H., Salzberg, S. L., Rinn, J. L., and Pachter, L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robinson, M. D., McCarthy, D. J., and Smyth, G. K. (2010) edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benjamini, Y., and Hochberg, Y. (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Royal Stat. Soc. 57, 289–300 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 51. Sun, L., Zhang, Z., Bailey, T. L., Perkins, A. C., Tallack, M. R., Xu, Z., and Liu, H. (2012) Prediction of novel long non-coding RNAs based on RNA-Seq data of mouse Klf1 knockout study. BMC Bioinformatics 13, 331 10.1186/1471-2105-13-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stover, C. K., De La Cruz, V. F., Fuerst, T. R., Burlein, J. E., Benson, L. A., Bennett, L. T., Bansal, G. P., Young, J. F., Lee, M. H., Hatfull, G. F., Snapper, S. B., Barletta, R. G., Jacobs, W. R., Jr., and Bloom, B. R. (1991) New use of BCG for recombinant vaccines. Nature 351, 456–460 10.1038/351456a0 [DOI] [PubMed] [Google Scholar]
- 53. Li, Y., Zeng, J., and He, Z. G. (2010) Characterization of a functional C-terminus of the Mycobacterium tuberculosis MtrA responsible for both DNA binding and interaction with its two-component partner protein, MtrB. J. Biol. Chem. 148, 549–556 10.1093/jb/mvq082 [DOI] [PubMed] [Google Scholar]
- 54. Padilla-Benavides, T., Long, J. E., Raimunda, D., Sassetti, C. M., and Argüello, J. M. (2013) A novel P1B-type Mn2+-transporting ATPase is required for secreted protein metallation in mycobacteria. J. Biol. Chem. 288, 11334–11347 10.1074/jbc.M112.448175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Colangeli, R., Haq, A., Arcus, V. L., Summers, E., Magliozzo, R. S., McBride, A., Mitra, A. K., Radjainia, M., Khajo, A., Jacobs, W. R., Salgame, P., and Alland, D. (2009) The multifunctional histone-like protein Lsr2 protects mycobacteria against reactive oxygen intermediates. Proc. Natl. Acad. Sci. U. S. A. 106, 4414–4418 10.1073/pnas.0810126106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang, J., Ge, P., Qiang, L., Tian, F., Zhao, D., Chai, Q., Zhu, M., Zhou, R., Meng, G., Iwakura, Y., Gao, G. F., and Liu, C. H. (2017) The mycobacterial phosphatase PtpA regulates the expression of host genes and promotes cell proliferation. Nat. Commun. 8, 244 10.1038/s41467-017-00279-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang, J., Li, B. X., Ge, P. P., Li, J., Wang, Q., Gao, G. F., Qiu, X. B., and Liu, C. H. (2015) Mycobacterium tuberculosis suppresses innate immunity by coopting the host ubiquitin system. Nat. Immunol. 16, 237–245 10.1038/ni.3096 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data described are presented either within the article or in the supporting information.