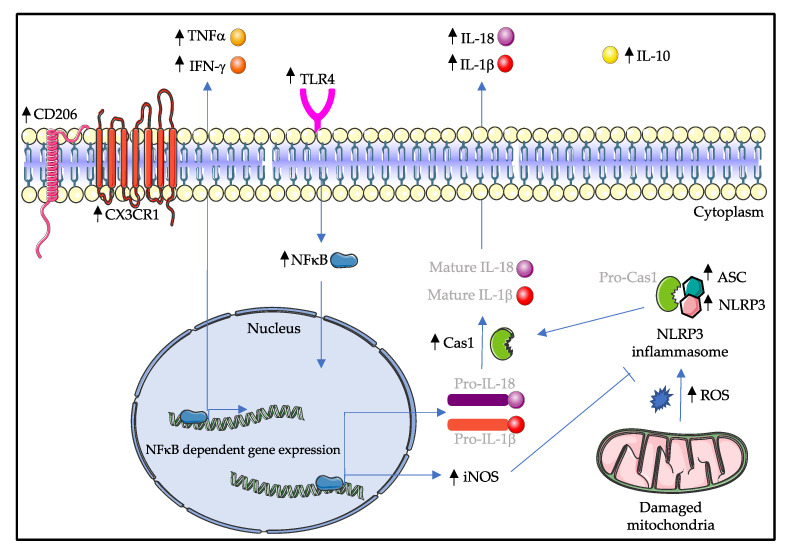
Figure 2.
Possible signaling cascades in the spinal cord microglia of the symptomatic SOD1G93A mice. Activation of TLR4 signaling induces the translocation of NFκB into the nucleus, where NFκB can upregulate the expression of M1-type proinflammatory cytokines such as TNFα, IFN-γ, iNOS, Pro-IL-1β and Pro-IL-18. Furthermore, increased production of reactive oxygen species by damaged mitochondria induces the activation of NLRP3 inflammasome, ultimately leading to the cleavage of IL-1β and IL-18 molecules. Surprisingly, M2-type microglia markers IL-10 and CD206 are upregulated. The balance between M1 and M2 activation states is broken down in symptomatic SOD1G93A mice, and M1-type microglia outweigh M2-type microglia, causing microgliosis and eventually motor neuron degeneration. Molecules that are written in light gray are not mentioned in this review. Black arrows indicate an increase in the amount of the respective molecule. CD206: cluster of differentiation 206; CX3CR1: C-X3-C motif chemokine receptor 1; TNFα: tumor necrosis factor alpha; IFN-γ: interferon gamma; TLR4: toll-like receptor 4; IL: interleukin; NFκB: nuclear factor kappa B; Cas1: caspase 1; iNOS: inducible nitric oxide synthase; ASC: apoptosis associated speck-like protein containing a CARD; NLRP3: nucleotide binding domain and leucine rich repeat containing protein 3; ROS: reactive oxygen species.