Figure 3.
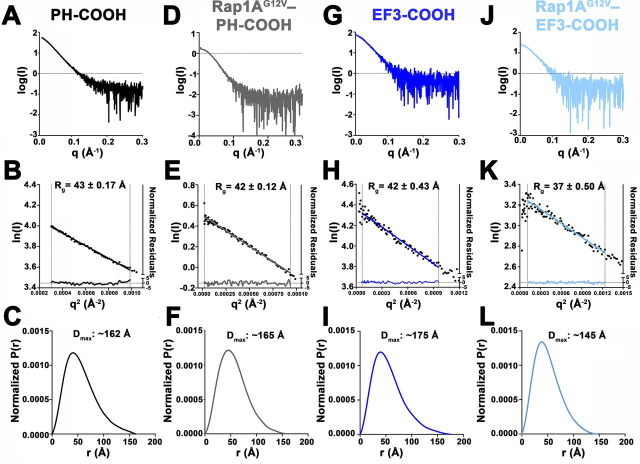
Rap1AG12V binding to PLCε PH-COOH or EF3-COOH stabilizes different conformational states.A and B, scattering profile for PLCε PH-COOH (A) and Guinier plot (B) demonstrate the variant is monomeric and monodisperse in solution. (C) Its pair–distance distribution function is consistent with a largely globular protein with some extended features. D and E, the scattering profile (D) and Guinier plot (E) for the Rap1AG12V–PH-COOH complex are also consistent with a monodisperse complex. (F) Its pair–distance distribution function shows a more compact structure upon the binding of Rap1AG12V. G–I, PLCε EF3-COOH is similar to PH-COOH in solution, as evidenced by its scattering profile (G), Guinier plot (H), and pair–distance distribution function (I). J and K, the Rap1AG12V–EF3-COOH complex does not have elevated lipase activity but is still monodisperse in solution as shown in (J) the scattering profile and (K) Guinier plot. (L) The shape of the pair–distance distribution function reveals the complex is more globular than EF3-COOH alone, and more compact, as evidenced by the smaller Dmax. The data for the PLCε PH-COOH variant are included for comparison (24).