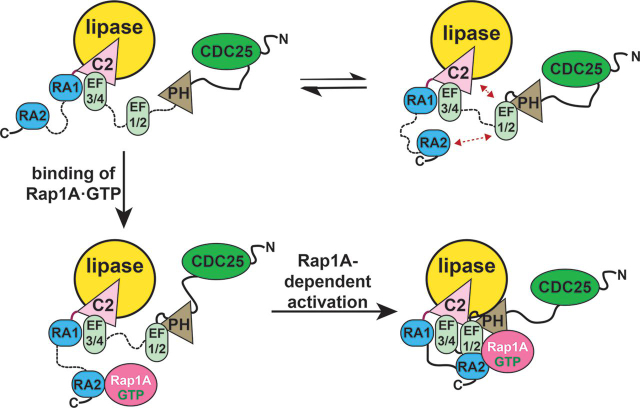
Figure 5.
A model for activation of PLCε by Rap1A.Top panels, PLCε exists in multiple conformational states in solution. The PH domain, EF1/2, and RA2 domains are flexibly connected to the rest of the enzyme, as indicated by the dashed black lines, and interact transiently with one another under basal conditions (red arrows) (24). Bottom left panel, activated Rap1A binds to its high-affinity binding site on the PLCε RA2 domain. However, this interaction is insufficient on its own to activate lipase activity. Bottom right panel, the Rap1A–RA2 complex also interacts with a site on the PLCε core, potentially formed by the PH domain and EF hands, resulting in Rap1A-dependent activation.