Figure 3.
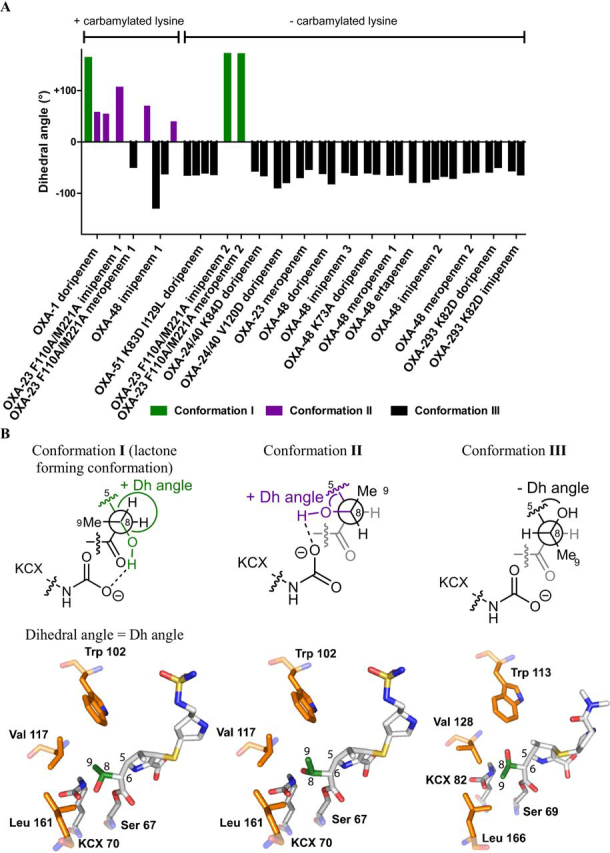
Crystallographically observed conformations of the carbapenem C-6 hydroxyethyl side chain.A, dihedral angles (Dh angles) of the hydroxyethyl side chain (O C-8 C-6 C-5) in the AECs of class D SBLs and carbapenems, in the presence and absence of a carbamylated lysine. The following structures were analyzed: OXA-1 with doripenem, pH 7.5 (PDB entry 3ISG) (19); OXA-23 F110A, M221A with imipenem, pH 7.0 (PDB entry 6N6X) (20); OXA-23 F110A, M221A with meropenem, pH 7.0 (PDB entry 6N6Y) (20); OXA-23 F110A, M221A with meropenem, pH 4.1 (PDB entry 6N6V) (20); OXA-23 F110A, M221A pH 4.1 with imipenem, (PDB entry 6N6U) (20); OXA-24/40 K84D with doripenem, pH 8.5 (PDB entry 3PAE) (24); OXA-24/40 V130D with doripenem, pH 8.5 (PDB entry 3PAG) (24); OXA-23 with meropenem, pH 4.1 (PDB entry 4JF4) (25); OXA-51 K83D, I129L with doripenem, pH 6.5 (PDB entry 5L2F) (26); OXA-13 with imipenem (PDB entry 1H5X) (28); OXA-48 with imipenem, pH 7.5 (1) (PDB entry 5QB4) (18); OXA-48 K73A with doripenem, pH 4.0 (PDB entry 6PXX) (30); OXA-48 with ertapenem, pH 4.0 (PDB entry 6P99) (32); OXA-48 with imipenem, pH 4.0 (3) (PDB entry 6P97) (32); OXA-48 with meropenem, pH 4.0 (1) (PDB entry 6P98) (32); OXA-48 with doripenem, pH 4.0 (PDB entry 6P9C) (32); OXA-48 with imipenem, pH 4.6 (2) (PDB entry 6PTU); OXA-48 with meropenem, pH 4.6 (2) (PDB entry 6PT1); OXA-239 K82D with doripenem, pH 4.2 (PDB entry 5WI7) (27); and OXA-239 K82D with imipenem, pH 4.2 (PDB entry 5WIB) (27). Numbers are used in cases in which there were more than one of the same enzyme: carba penem crystal structure. This analysis suggests that the carbamylated lysine is an important determinant of the C-6 hydroxyethyl side chain conformation. B, view of hydroxyethyl conformations in the OXA-1 active site with doripenem (conformations I and II) and the OXA-23 F110A/M221A active site with meropenem (conformation III), showing dihedral angles and interactions of the carbamylated lysine and surrounding residues with the C-6 hydroxyethyl side chain. Conformation I is proposed to most closely represent the conformation required for β-lactone formation, because the C-6 hydroxyethyl hydroxyl group is positioned in a suitable orientation (i.e. has a favorable Bürgi–Dunitz trajectory) relative to the AEC carbonyl. Note, Trp113/Val128/Leu166 in OXA-23 and Trp102/Val117/Leu161 in OXA-1 are equivalent to Trp105/Val120/Leu158 in OXA-48. KCX 70, carbamylated Lys70.
