Figure 2.
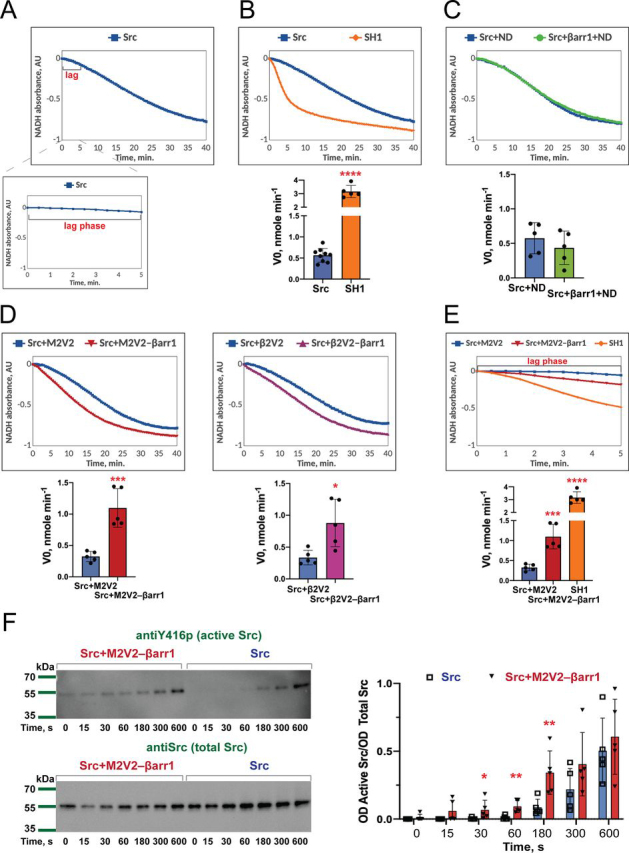
GPCR-activated βarr 1 allosterically activates Src in vitro by reducing the lag phase in enzyme activation.A, lag phase in Src activation: representative progress curve of NADH oxidation coupled to peptide phosphorylation by Src measured by continuous kinase colorimetric assay shown over the course 40 min (top panel) and during the first 5 min of reaction (bottom panel). Optimal Src peptide (AEEEIYGEFEAKKKK) is used at a concentration of 250 μm, Src was used at a concentration of 25 nm. B, constitutively active Src (SH1) shows no lag phase. Top panel: representative progress curves of NADH oxidation coupled to peptide phosphorylation by Src and SH1 measured by continuous kinase colorimetric assay. Bottom panel: initial velocity of peptide phosphorylation (V0) by Src and SH1. Individual data show mean ± S.D. of five independent experiments (Src: 9 independent experiments). Statistical differences were determined by one-way ANOVA and Dunnett's multiple comparison test (****, p < 0.0001). Optimal Src peptide (AEEEIYGEFEAKKKK) is used at a concentration of 250 μm, Src and SH1 were used at a concentration of 25 nM. C, βarr1 alone does not have an effect on Src activity. Top panel: representative progress curves of NADH oxidation coupled to peptide phosphorylation by Src as measured by continuous kinase colorimetric assay. Bottom panel: initial velocity of peptide phosphorylation (V0). Individual data show mean ± S.D. of five independent experiments. Statistical differences were determined by one-way ANOVA and Dunnett's multiple comparison test. Optimal Src peptide (AEEEIYGEFEAKKKK) is used at a concentration of 250 μm, Src was used at a concentration of 25 nm, βarr1 was used at a concentration of 125 nm. To reproduce the exact conditions of the experiment with GPCR–βarr1 complexes, empty MSP1D1E3 (ND) nanodisc and Fab30 were added to Src at a concentration of 125 nm. D, M2V2–βarr1 and β2V2–βarr1 complexes activate Src in vitro. Top panel: representative progress curves of NADH oxidation coupled to peptide phosphorylation by Src as measured by continuous kinase colorimetric assay. Bottom panel: initial velocity of peptide phosphorylation (V0). Individual data show mean ± S.D. of five independent experiments. Statistical differences were determined by one-way ANOVA and Dunnett's multiple comparison test (*, p < 0.05; ***, p < 0.0005). Optimal Src peptide (AEEEIYGEFEAKKKK) was used at a concentration of 250 μm and Src was used at a concentration of 25 nm. M2V2–βarr1 and β2V2–βarr1 are used at a concentration of 125 nm. M2V2–βarr1 and β2V2–βarr1 complexes are additionally stabilized by a synthetic antibody fragment Fab30 (125 nm). To reproduce the exact conditions of the experiment with GPCR–βarr1 complexes, M2V2/β2V2 and Fab30 were added to Src at a concentration of 125 nm. M2V2 was activated by iperoxo, β2V2 was activated by BI-167107. E, GPCR-activated βarr1 reduces the lag phase in enzyme activity: Top panel: representative progress curves of NADH oxidation coupled to peptide phosphorylation by Src as measured by continuous kinase colorimetric assay during first 5 min of reaction. Bottom panel: initial velocity of peptide phosphorylation (V0). Optimal Src peptide (AEEEIYGEFEAKKKK) is used at a concentration of 250 μm, Src and SH1 were used at a concentration of 25 nm. M2V2–βarr1 is used at a concentration of 125 nm. M2V2-βarr1 complex is additionally stabilized by a synthetic antibody fragment Fab30 (125 nm). M2V2 was activated by iperoxo. To reproduce the exact conditions of the experiment with GPCR–βarr1 complexes, M2V2/β2V2 and Fab30 were added to Src at a concentration of 125 nm. M2V2 was activated by iperoxo, β2V2 was activated by BI-167107. F, M2V2–βarr1 complex promotes Src autophosphorylation. Left panel: time course of activation loop Tyr-416 autophosphorylation of Src in vitro. Representative Western blots are shown. Right panel: densitometry analysis of Tyr-416 phosphorylation expressed as a ratio over total Src (optical density of active Src/OD total Src). Src was used at a concentration of 12.5 nm and M2V2–βarr1–Fab complexes were used at a concentration of 125 nM. Individual data show mean ± S.D. of five independent experiments. Statistical differences were determined by Mann-Whitney test (*, p < 0.05; **, p < 0.01 as compared with the corresponding time point of Src alone reaction).
