Figure 3.
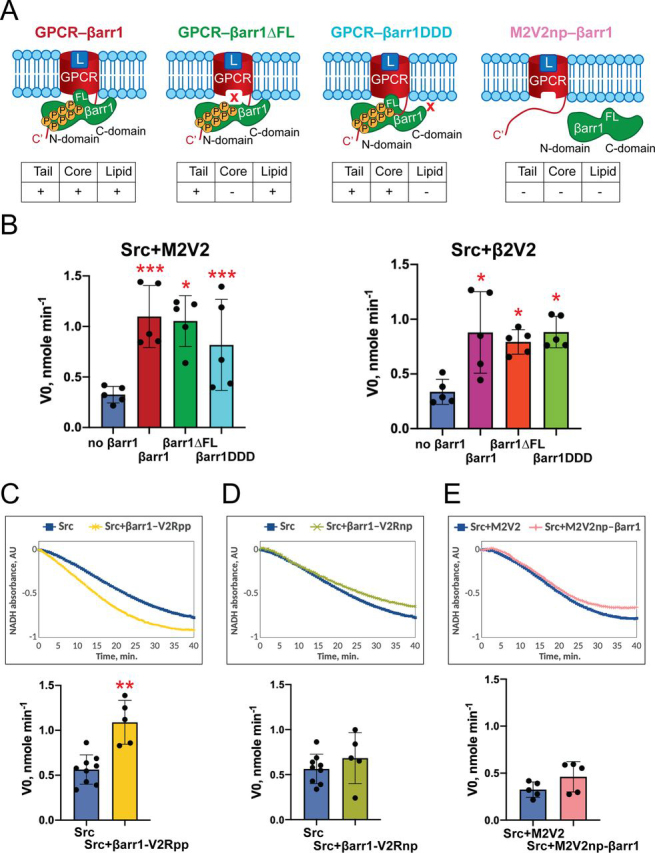
Phosphorylated GPCR tail interaction with β-arrestin 1 is sufficient to confer the activation of Src.A, cartoon illustrating the effect of βarr1 mutations and receptor C-terminal tail phosphorylation on the conformation of GPCR–βarr1 complexes. GPCR–βarr1 complexes are additionally stabilized by a synthetic antibody fragment Fab30 (not shown for clarity) (L, ligand; FL, finger loop). B, interactions of βarr1 with the receptor core and with the lipid are dispensable for activation of Src. Initial velocity of peptide phosphorylation by Src as measured by continuous kinase colorimetric assay. Individual data show mean ± S.D. of five independent experiments. Statistical differences were determined by one-way ANOVA and Dunnett's multiple comparison test (*, p < 0.05; ***, p < 0.0005). Optimal Src peptide (AEEEIYGEFEAKKKK) was used at a concentration of 250 μm and Src used at a concentration of 25 nm. M2V2–βarr1 and β2V2–βarr1 are used at a concentration of 125 nm. M2V2–βarr1 and β2V2–βarr1 complexes are additionally stabilized by a synthetic antibody fragment Fab30 (125 nm). To reproduce the exact conditions of the experiment with GPCR–βarr1 complexes, M2V2/β2V2 and Fab30 were added to Src at a concentration of 125 nm. M2V2 was activated by iperoxo, β2V2 was activated by BI-167107. C–E, receptor C-terminal tail phosphorylation is required for allosteric activation of Src. Representative progress curves of NADH oxidation coupled to peptide phosphorylation by Src, as measured by continuous kinase colorimetric assay (top panel) and initial velocity of peptide phosphorylation by Src (bottom panel), in the presence of βarr1–V2Rpp (C), βarr1–V2Rnp (D), or M2V2np–βarr1 (E) are shown. Individual data show mean ± S.D. of five independent experiments (Src: 9 independent experiments). Statistical differences were determined by one-way ANOVA and Dunnett's multiple comparison test (**, p < 0.01). Optimal Src peptide (AEEEIYGEFEAKKKK) is used at a concentration of 250 μm and Src was used at a concentration of 25 nm. βarr1–V2Rpp, M2V2np, βarr1, V2Rnp, and Fab30 are added at a concentration of 125 nm. To reproduce the exact conditions of the experiment with GPCR–βarr1 complexes, M2V2 and Fab30 were added to Src at a concentration of 125 nm. M2V2np was activated by iperoxo.
