Abstract
Background: Young adults with type 1 diabetes (T1D) tend to have higher A1C than older adults and are at increased risk for diabetic ketoacidosis (DKA). Oral adjuncts to insulin have not been previously studied in this population.
Methods: In this phase 2, multicenter, randomized, double-blind, placebo-controlled, parallel-group study, adults aged 18–30 years with T1D and A1C ≥9.0% were randomly assigned to placebo (n = 42) or sotagliflozin 400 mg (n = 43), in addition to insulin for 12 weeks. Insulin doses were adjusted to meet glucose targets (preprandial 80–130 mg/dL, postprandial <180 mg/dL). The primary endpoint was change from baseline in A1C at week 12.
Results: From a baseline of 9.8%, mean A1C decreased by 1.0% with placebo and 1.3% with sotagliflozin (−0.4% [95% confidence interval (CI): −0.8 to 0.1]; P = 0.10 vs. placebo). In the prespecified A1C ≤10.0% subgroup, the treatment difference was −0.8% (−1.3 to −0.2; P = 0.006), favoring sotagliflozin. Overall, relative to placebo, postprandial glucose (PPG) decreased by 56.6 mg/dL (−89.7 to −23.6; P < 0.001) and weight decreased by 2.37 kg (−3.5 to −1.2; P < 0.001). More patients achieved an A1C <7.0% with sotagliflozin (16.3%) than placebo (2.4%; P = 0.026). Rates of documented hypoglycemia and severe hypoglycemia were similar between groups. One DKA event occurred with placebo, and none occurred with sotagliflozin.
Conclusions: In young adults with T1D and suboptimal glycemic control, sotagliflozin plus insulin for 12 weeks numerically improved A1C and significantly improved A1C goal attainment, PPG, and body weight. Sotagliflozin plus insulin was generally well tolerated without any episodes of DKA (NCT02383940).
Keywords: Sotagliflozin, Type 1 diabetes, Adjunctive therapy, Young adults, Diabetic ketoacidosis, SGLT inhibitors
Introduction
Teens and young adults with type 1 diabetes (T1D) frequently have poor glycemic control and experience the highest incidence of diabetic ketoacidosis (DKA). In the latest report from the T1D Exchange, the mean A1C among patients 18–25 years was >8.5%, and 4% of teens and young adults experienced a DKA event within the preceding 3 months compared with 1%–2% in older age groups.1 Poor glycemic control and increased DKA risk in youth and young adults have been attributed to various factors, including stigma and peer pressure to engage in high-risk behavior, the transition from parental control of diabetes management to self-management, stress coupled with low resilience and low self-esteem, and a failure to connect behavioral choices to health outcomes.2–4
Adjunctive therapy with an oral antihyperglycemic agent might offer younger patients with T1D a convenient means of achieving better control of blood glucose, but this question has not been previously examined in clinical trials. Before 2019, the amylin analog pramlintide was the only agent approved as an insulin adjunct in T1D, but the need for multiple daily injections and an increased risk of severe hypoglycemia have limited use of this agent.5–7 Recently, sodium-glucose cotransporter (SGLT) inhibitors have been evaluated as an adjunct therapy to insulin in adults with T1D.8–13 The SGLT inhibitors were associated with significant improvements in glycemic control, body weight, and blood pressure with no increased risk of hypoglycemia, but with an increased risk of DKA.
Although adults older than 18 years were eligible for inclusion in these studies, the mean age of participants ranged from 41 to 46 years,8–13 leaving a gap in understanding of the effect of SGLT inhibitors plus insulin in the subpopulation of younger adults with T1D. Given the difficulties of managing diabetes in this younger population with poor glucose control and greater risk of DKA, this phase 2, randomized, placebo-controlled study examined the efficacy and safety of sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, in patients aged 18–30 years with poorly controlled T1D (i.e., A1C ≥9%). This study was cosponsored by the Juvenile Diabetes Research Foundation (JDRF) and Lexicon Pharmaceuticals, Inc.
Research Design and Methods
This phase 2, multicenter, randomized, double-blind, placebo-controlled, parallel-group study was conducted at 15 sites in the United States and evaluated the efficacy of 12 weeks of oral sotagliflozin 400 mg once daily in combination with insulin in patients aged 18–30 years with T1D and an A1C ≥9.0% at screening. Randomization was stratified by insulin delivery with either multiple daily insulin injections (MDI) or continuous subcutaneous insulin infusion (CSII) and by screening A1C (≤10.0% and >10.0%). To qualify for randomization, patients had to demonstrate ≥80% compliance with taking the expected amount of placebo tablets during a 2-week placebo run-in period and checking 5-point self-monitored blood glucose (SMBG) as required by the protocol.
Institutional Review Boards (IRBs) approved the protocol and consent forms. All patients provided written informed consent. An independent clinical endpoint committee, blinded to trial treatment, adjudicated deaths and events of special interest, including major adverse cardiovascular events, drug-induced liver injury, DKA, and severe hypoglycemia. An independent data monitoring committee reviewed trial conduct and patient safety. An independent statistician performed statistical analysis. This trial was registered with clinicaltrials.gov (NCT02383940).
Study population
Eligible patients were ≥18 and ≤30 years of age with T1D treated with intensive insulin therapy (MDI or CSII) for ≥1 year and had an A1C at screening ≥9.0%. In addition, patients were required to have an estimated glomerular filtration rate (eGFR) ≥45 mL/min/1.73 m2. Complete inclusion and exclusion criteria appear in the Supplementary Data.
Interventions and procedures
After a 2-week placebo run-in period, patients were randomly assigned in a 1:1 ratio to placebo or sotagliflozin 400 mg, given as two 200-mg tablets administered orally once daily. Insulin doses were adjusted by investigators to meet recommended targets of A1C <7.0%, fasting or preprandial blood glucose between 80 and 130 mg/dL, and 2-h peak postprandial glucose (PPG) ≤180 mg/dL based on SMBG patterns as well as fasting plasma glucose (FPG) and A1C data collected by investigators. Investigators were permitted to adjust glycemic targets at their discretion to meet the needs of individual patients. No protocol-specific instructions regarding diet, exercise, or other lifestyle measures were provided.
The morning of day 1, before administration of study medication, all patients consumed a standardized mixed meal for breakfast and had blood samples drawn 2 h later to evaluate PPG levels at baseline. The 2-h standardized mixed-meal procedure was repeated during the last visit at week 12 after administration of the study drug (see Supplementary Data for details). The study medication was initiated before lunch on day 1 (after completion of the 2-h PPG test), and bolus insulin was reduced by 30% for this meal on day 1 only.14
As detailed in the phase 3 sotagliflozin trial reports (in Tandem1, 2, and 3),8–10 all patients received urine ketone strips and blood β-hydroxybutyrate (BHB) meters and strips as well as instructions for detecting and treating ketosis, urogenital hygiene, and proper hydration. Patients also received a card with DKA detection and treatment information, which included an emergency contact phone number for their study center (see Supplementary Data for details). Study centers received recommendations for ketosis and DKA diagnosis and management. Key elements of these recommendations included instructions on the identification, diagnosis, and treatment of ketosis and DKA and on withholding the study drug before scheduled surgeries or procedures requiring withholding of oral intake (see Supplementary Data for details).
All patients were instructed to perform 5-point SMBG at least 5 days per week before each visit. All patients wore a blinded CGM device (Dexcom G4; Dexcom, Inc., San Diego, CA) for specified 7-day periods, including the week before day 1 (baseline) and the 2 weeks before the final study visit. A minimum of 3 days' worth of valid recordings was required for the CGM data to be considered valid for analysis.
Endpoints
The primary endpoint was the change in least squares mean (LSM) A1C from baseline to week 12. Secondary endpoints were the LSM changes from baseline to week 12 in daily bolus insulin, 2-h PPG after a standardized mixed-meal tolerance test, CGM area under the curve (AUC) over 24 h by hyperglycemia (>150 mg/dL) and hypoglycemia (<70 mg/dL), SMBG-documented hypoglycemia (≤70 mg/dL), and daily basal insulin.
Total, basal, and bolus insulin doses were also evaluated during weeks 1, 12, and 13. A composite net clinical benefit endpoint described the proportion of patients with A1C <7.0% at week 12 without any recorded episodes of DKA or severe hypoglycemia during the 12-week treatment period. Additional endpoints included A1C change from baseline in specific subgroups (CSII or MDI and A1C ≤10.0% or >10.0%) and changes from baseline in body weight, FPG, mean daily glucose via CGM, and eGFR. An exploratory endpoint was the percentage of time in range (70–180 mg/dL) by CGM glucose (assuming 100% daily CGM data available for analysis, 1.0% of daily CGM time was considered equivalent to 0.24 h).
Safety endpoints included clinically significant laboratory results, adverse events (AEs), and severe AEs. Events of special interest included documented or severe hypoglycemia, DKA, metabolic acidosis, volume depletion, major adverse cardiovascular events, genital mycotic infection, urinary tract infection, diarrhea, pancreatitis, bone fractures, events leading to amputation, venous thrombotic events, drug-induced liver injuries, renal events, malignancy, and deaths. The number of hypoglycemic events per day was calculated as a daily average number of episodes of SMBG-confirmed glucose ≤70 mg/dL over the week before a visit.
Statistical methods
All efficacy analyses were conducted using the modified intent-to-treat (mITT) population, defined as all randomized patients who had taken at least 1 dose of study drug. Supportive analyses were conducted with the per-protocol population, defined as mITT patients who completed study treatment through the primary efficacy assessment of 12 weeks and who had no significant protocol deviations that would have a major impact on the collection or interpretation of the primary efficacy endpoint.
Analysis of the primary efficacy endpoint used the mixed-effect model for repeated measures (MMRM) statistics based on the restricted maximum likelihood method for estimation. The analysis model included fixed, categorical effects of treatment, insulin delivery method (CSII, MDI), screening A1C (≤10%, >10%), time (study week), baseline-A1C-by-time interaction, and a treatment-by-time interaction. Only post-baseline, scheduled study visits for A1C up to week 12 were included in the MMRM. An unstructured (co)variance structure was used to model the within-patient errors. The adjusted mean change in A1C from baseline to week 12 for each treatment group and the 95% confidence intervals (CIs) were estimated in the framework of this model, as well as the between-group difference and the 95% CIs for the difference. Analysis visit windows were applied to all observations, including data collected after the discontinuation of study drug, to determine the values to be used in the MMRM.
The summary of inferential statistics included LSM, standard error (SE) of the estimates, P values, and 2-sided CIs. For continuous endpoints, MMRM or analysis of covariance (ANCOVA) models, with the corresponding baseline value (interaction with time, if MMRM) in the model, were used to compare the treatment groups as appropriate. The choice in statistical models depended on whether the measurement process was over multiple time points (MMRM, including scheduled study visits/weeks up to week 12 only) or at a single time point (ANCOVA). For binary endpoints, a Cochran–Mantel–Haenszel test stratified by the insulin delivery method and screening A1C was used.
Results
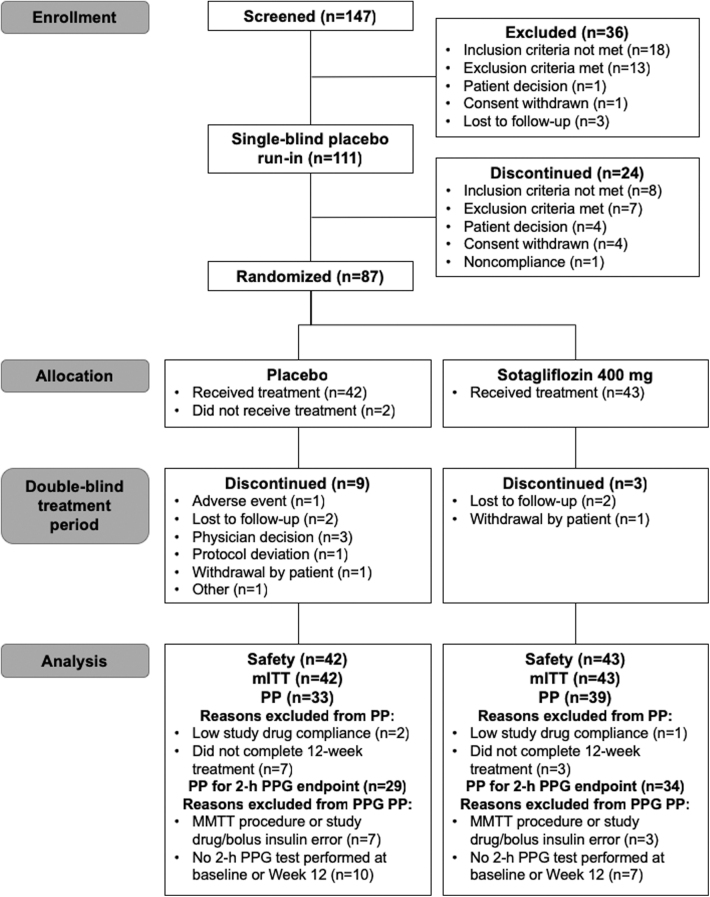
Beginning April 2015 through September 2016, 147 young adults with T1D were screened, 111 entered the placebo run-in, and 87 were randomly assigned to treatment. Two patients randomized to the placebo group did not receive the study drug, so analyses were performed in the 42 patients assigned to placebo and 43 to sotagliflozin 400 mg. Nine patients in the placebo and three patients in the sotagliflozin group discontinued study treatment (Fig. 1). Majority of patients were white, and the average age was 22 years. The mean duration of diabetes was 12 years, and 54.1% used CSII. Overall, patients had a baseline A1C of 9.8%, and 56.5% had an A1C >10.0% (Table 1). Baseline demographics were generally similar between treatment groups with the exception of documented hypoglycemia via SMBG during the screening period, which was twice as frequent in the placebo group (0.3 ± 0.4 and 0.1 ± 0.2 events/patient per day in the placebo and sotagliflozin groups, respectively; Table 1).
FIG. 1.
Patient disposition. mITT, modified intent to treat; MMTT, mixed-meal tolerance test; PP, per protocol; PPG, postprandial glucose.
Table 1.
Baseline Demographics and Characteristics
| Characteristic | Placebo (n = 42) | Sotagliflozin 400 mg (n = 43) | Total (N = 85) |
|---|---|---|---|
| Mean age, years ± SD | 21.7 ± 3.6 | 22.8 ± 4.0 | 22.3 ± 3.8 |
| Female, n (%) | 23 (54.8) | 22 (51.2) | 45 (52.9) |
| Race, n (%) | |||
| Asian | 1 (2.4) | 0 | 1 (1.2) |
| Black | 6 (14.3) | 2 (4.7) | 8 (9.4) |
| White | 34 (81.0) | 41 (95.3) | 75 (88.2) |
| Other | 1 (2.4) | 0 | 1 (1.2) |
| Body weight, kg ± SD | 77.3 ± 14.6 | 83.7 ± 20.4 | 80.5 ± 18.0 |
| BMI, kg/m2 ± SD | 26.7 ± 5.0 | 29.4 ± 7.2 | 28.1 ± 6.3 |
| BMI ≥30 kg/m2, n (%) | 10 (23.8) | 18 (41.9) | 28 (32.9) |
| T1D duration, years ± SD | 11.9 ± 5.4 | 11.9 ± 6.2 | 11.9 ± 5.8 |
| Insulin delivery method, n (%) | |||
| CSII | 23 (54.8) | 23 (53.5) | 46 (54.1) |
| MDI | 19 (45.2) | 20 (46.5) | 39 (45.9) |
| Total daily insulin, IU/kg ± SD | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 |
| Ratio of bolus to total insulin ± SD | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 |
| A1C, % ± SD | 9.7 ± 0.9 | 9.9 ± 1.4 | 9.8 ± 1.2 |
| A1C, mmol/mol ± SD | 82.8 ± 10.2 | 85.1 ± 15.4 | 84.0 ± 13.1 |
| A1C at screening, n (%) | |||
| ≤10.0% | 19 (45.2) | 18 (41.9) | 37 (43.5) |
| >10.0% | 23 (54.8) | 25 (58.1) | 48 (56.5) |
| 2-h PPG, mg/dL ± SD | 208.8 ± 81.0 | 239.4 ± 96.3 | 224.1 ± 89.7 |
| FPG, mg/dL ± SD | 187.1 ± 75.6 | 199.2 ± 83.8 | 193.2 ± 79.6 |
| BHB (mmol/L) ± SD | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 |
| eGFR, mL/min/1.73 m2 ± SD | 114.5 ± 19.8 | 113.2 ± 22.6 | 113.9 ± 21.1 |
| Documented hypoglycemia (SMBG <70 mg/dL), events/patient per day ± SDa | 0.3 ± 0.4 | 0.1 ± 0.2 | 0.2 ± 0.3 |
The number of hypoglycemic events per day was calculated as a daily average number of episodes over the week before a visit.
BHB, β-hydroxybutyrate; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; MDI, multiple daily insulin injections; PPG, postprandial glucose; SD, standard deviation; SMBG, self-monitored blood glucose; T1D, type 1 diabetes.
Glucose control
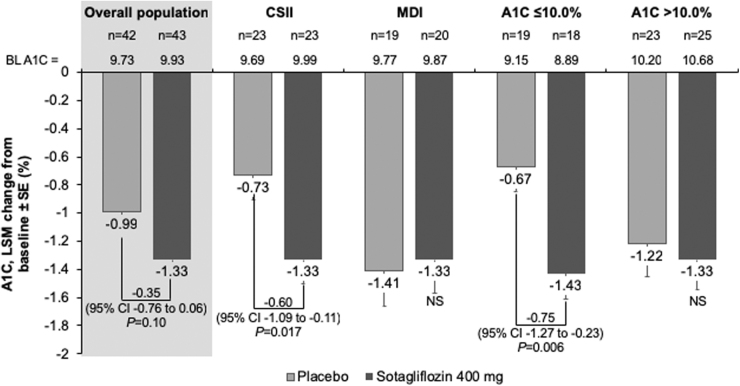
As shown in Table 2 and Figure 2, LSM A1C change from baseline was −1.0 ± 0.1 with placebo and −1.3 ± 0.1 with sotagliflozin (between-group treatment difference −0.4% [95% CI: −0.8 to 0.1; P = 0.10]). In prespecified analyses, the placebo-adjusted A1C change from baseline was −0.8% (95% CI: −1.3 to −0.2; P = 0.006) with sotagliflozin in the subgroup with A1C ≤10.0% and −0.1% (95% CI: −0.7 to 0.5; P = 0.73) in patients with A1C >10.0% at screening. The placebo-adjusted changes from baseline in A1C were −0.6% (95% CI: −1.1 to −0.1; P = 0.017) and 0.1 (95% CI: −0.6 to 0.8; P = 0.83) in the subgroups of patients receiving insulin via CSII and MDI, respectively.
Table 2.
Primary, Secondary, and Other Efficacy Endpoints in the Modified Intent-to-Treat Population
| Endpoint | Placebo (n = 42) | Sotagliflozin 400 mg (n = 43) |
|---|---|---|
| A1C (%) | ||
| Baseline, mean ± SD | 9.7 ± 0.9 | 9.9 ± 1.4 |
| No. of patients, week 12 | 34 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | −1.0 ± 0.1 (−1.3 to −0.7); <0.001 | −1.3 ± 0.1 (−1.6 to −1.1); <0.001 |
| Difference from placebo, LSM ± SE (95% CI); P value | −0.4 ± 0.2 (−0.8 to 0.1); 0.10 | |
| A1C (mmol/mol) | ||
| Baseline, mean ± SD | 89.5 ± 10.2 | 92.7 ± 14.2 |
| No. of patients, week 12 | 34 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | −10.6 ± 1.6 (−13.8 to −7.3); <0.001 | −14.5 ± 1.6 (−17.7 to −11.4); <0.001 |
| Difference from placebo, LSM ± SE (95% CI); P value | −4.0 ± 2.3 (−8.5 to 0.6); 0.08 | |
| 2-h PPG (mg/dL) | ||
| Baseline, mean ± SD | 208.8 ± 81.0 | 239.4 ± 96.3 |
| No. of patients, week 12 | 32 | 36 |
| Difference from baseline, LSM ± SE (95% CI); P value | 0.2 ± 12.2 (−24.2 to 24.7); 0.99 | −56.4 ± 11.6 (−79.6 to −33.2); <0.001 |
| Difference from placebo, LSM ± SE (95% CI); P value | −56.6 ± 16.6 (−89.7 to −23.6); 0.001 | |
| FPG (mg/dL) | ||
| Baseline, mean ± SD | 187.1 ± 75.6 | 199.2 ± 83.8 |
| No. of patients, week 12 | 34 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | −2.7 ± 13.0 (−28.6 to 23.2); 0.83 | −26.8 ± 12.1 (−51.0 to −2.6); 0.030 |
| Difference from placebo, LSM ± SE (95% CI); P value | −24.1 ± 17.8 (−59.5 to 11.3); 0.18 | |
| CGM AUC >150 mg/dL (mg/dL × min/1000) | ||
| Baseline, mean ± SD | 118.6 ± 47.5 | 126.8 ± 61.5 |
| No. of patients, week 12 | 28 | 27 |
| Difference from baseline, LSM ± SE (95% CI); P value | −5.0 ± 7.9 (−20.9 to 10.9); 0.53 | −27.3 ± 8.0 (−43.4 to −11.2); 0.001 |
| Difference from placebo, LSM ± SE (95% CI); P value | −22.3 ± 11.2 (−44.8 to 0.2); 0.052 | |
| CGM AUC <70 mg/dL (mg/dL × min/1000) | ||
| Baseline, mean ± SD | 1.0 ± 2.0 | 0.7 ± 1.1 |
| No. of patients, week 12 | 28 | 27 |
| Difference from baseline, LSM ± SE (95% CI); P value | 0.2 ± 0.3 (−0.3 to 0.7); 0.38 | 0.4 ± 0.3 (−0.1 to 0.9); 0.10 |
| Difference from placebo, LSM ± SE (95% CI); P value | 0.2 ± 0.4 (−0.5 to 0.9); 0.56 | |
| CGM time in range, ≥70 and ≤180 mg/dL (%) | ||
| Baseline, mean ± SD | 33.5 ± 13.2 | 33.1 ± 16.5 |
| No. of patients, week 12 | 28 | 27 |
| Difference from baseline, LSM ± SE (95% CI); P value | 1.4 ± 2.8 (−4.2 to 7.1); 0.61 | 9.1 ± 2.8 (3.5 to 14.7); 0.002 |
| Difference from placebo, LSM ± SE (95% CI); P value | 7.7 ± 3.9 (−0.2 to 15.6); 0.057 | |
| Bolus insulin dose (IU/day) | ||
| Baseline, mean ± SD | 30.1 ± 13.5 | 32.4 ± 17.6 |
| No. of patients, week 12 | 34 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | −3.0 ± 1.9 (−6.9 to 0.9); 0.13 | −4.9 ± 1.8 (−8.6 to −1.2); 0.010 |
| Difference from placebo, LSM ± SE (95% CI); P value | −1.9 ± 2.7 (−7.3 to 3.4); 0.47 | |
| Percent difference from baseline, LSM ± SE (95% CI); P value | −2.4 ± 9.3 (−20.9 to 16.1); 0.80 | −8.0 ± 8.7 (−25.4 to 9.3); 0.36 |
| Percent difference from placebo, LSM ± SE (95% CI); P value | −5.6 ± 12.7 (−30.9 to 19.6); 0.66 | |
| Total daily insulin dose (IU/day) | ||
| Baseline, mean ± SD | 67.8 ± 30.1 | 69.6 ± 26.9 |
| No. of patients, week 12 | 34 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | 1.0 ± 2.6 (−4.3 to 6.3); 0.70 | −2.9 ± 2.5 (−7.9 to 2.1); 0.25 |
| Difference from placebo, LSM ± SE (95% CI); P value | −3.9 ± 3.6 (−11.2 to 3.4); 0.29 | |
| Percent difference from baseline, LSM ± SE (95% CI); P value | 2.9 ± 4.0 (−5.1 to 10.9); 0.48 | −3.8 ± 3.8 (−11.4 to 3.7); 0.32 |
| Percent difference from placebo, LSM ± SE (95% CI); P value | −6.7 ± 5.5 (−17.7 to 4.3); 0.23 | |
| Basal insulin dose (IU/day) | ||
| Baseline, mean ± SD | 38.0 ± 23.6 | 37.1 ± 17.0 |
| No. of patients, week 12 | 34 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | 3.3 ± 1.3 (0.8 to 5.8); 0.012 | 2.0 ± 1.2 (−0.4 to 4.4); 0.10 |
| Difference from placebo, LSM ± SE (95% CI); P value | −1.2 ± 1.7 (−4.7 ± 2.3); 0.48 | |
| Percent difference from baseline, LSM ± SE (95% CI); P value | 11.1 ± 3.2 (4.7 to 17.5); <0.001 | 6.4 ± 3.1 (0.2 to 12.5); 0.043 |
| Percent difference from placebo, LSM ± SE (95% CI); P value | −4.7 ± 4.5 (−13.6 to 4.2); 0.29 | |
| Body weight (kg) | ||
| Baseline, mean ± SD | 77.3 ± 14.6 | 83.7 ± 20.4 |
| No. of patients, week 12 | 34 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | 1.8 ± 0.4 (0.9 to 2.6); <0.001 | −0.6 ± 0.4 (−1.4 to 0.2); 0.12 |
| Difference from placebo, LSM ± SE (95% CI); P value | −2.4 ± 0.6 (−3.5 to −1.2); <0.001 | |
| eGFR (mL/min/1.73 m2) | ||
| Baseline, mean ± SD | 114.5 ± 19.8 | 113.2 ± 22.6 |
| No. of patients, week 12 | 35 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | −1.1 ± 2.3 (−5.6 to 3.4); 0.61 | −4.4 ± 2.1 (−8.7 to −0.1); 0.044 |
| Difference from placebo, LSM ± SE (95% CI); P value | −3.3 ± 3.1 (−9.4 to 2.9); 0.30 | |
| Documented blood glucose ≤70 mg/dL (events/patient/day)a | ||
| Baseline, mean ± SD | 0.3 ± 0.4 | 0.1 ± 0.2 |
| No. of patients, week 12 | 35 | 40 |
| Difference from baseline, LSM ± SE (95% CI); P value | −0.04 ± 0.04 (−0.1 to 0.04); 0.31 | −0.001 ± 0.04 (−0.1 to 0.1); 0.98 |
| Difference from placebo, LSM ± SE (95% CI); P value | 0.04 ± 0.1 (−0.1 to 0.2); 0.47 | |
The number of hypoglycemic events per day was calculated as a daily average number of episodes over the week before a visit.
AUC, area under the curve; CGM, continuous glucose monitor; CI, confidence interval; LSM, least squares mean; SE, standard error.
FIG. 2.
LSM change from baseline to week 12 in A1C in the overall mITT population and prespecified subgroups. CI, confidence interval; CSII, continuous subcutaneous insulin infusion; LSM, least squares mean; MDI, multiple daily insulin injections; NS, not statistically significant; SE, standard error.
Relative to placebo, LSM 2-h PPG decreased by 56.6 mg/dL (95% CI: −89.7 to −23.6; P < 0.001) with sotagliflozin in the mITT population (Table 2). At 12 weeks, changes from baseline in FPG were −2.7 ± 13.0 mg/dL with placebo (P = 0.83 vs. baseline) and −26.8 ± 12.1 mg/dL with sotagliflozin (P = 0.030 vs. baseline), with a treatment difference of −24.1 mg/dL (95% CI: −59.5 to 11.3; P = 0.18; Table 2).
CGM AUC >150 mg/dL decreased by 22.3 mg/dL × min/1000 (95% CI: −44.8 to 0.2; P = 0.052) with sotagliflozin relative to placebo (Table 2). The placebo-adjusted change in CGM mean daily glucose was −17.8 mg/dL (95% CI: −37.2 to 1.6; P = 0.07). There was no between-group difference in CGM AUC in the hypoglycemic range (<70 mg/dL; Table 2), nor was there a between-group difference in the change from baseline in the number of fingerstick readings ≤70 mg/dL per person per day. In an exploratory analysis, the percentage of CGM time in range (70–180 mg/dL) increased by 7.7% ± 3.9%, or 1.8 h, with sotagliflozin relative to placebo (P = 0.057).
Insulin dose
The differences between sotagliflozin and placebo in bolus, basal, and total daily insulin doses at week 12 were −5.6% (95% CI: −30.9 to 19.6; P = 0.66), −4.7% (95% CI: −13.6 to 4.2; P = 0.29), and −6.7% (95% CI: −17.7 to 4.3; P = 0.23), respectively (Table 2). In subgroup analyses of patients using MDI or CSII, there were no significant differences between treatment groups in basal, bolus, or total insulin doses. However, among placebo recipients using MDI, mean daily basal insulin doses rose steadily from baseline over the treatment period, with a 15.0% increase by week 12 (95% CI: 2.0 to 27.9); whereas basal insulin doses remained relatively stable among sotagliflozin recipients using MDI (+5.8% at week 12 [95% CI: −6.4 to 18.0]).
Target A1C goal
From an average baseline A1C of ∼9.8% observed for the full randomized patient sample, more patients achieved an A1C <7.0% with sotagliflozin (16.3%) compared with placebo (2.4%), for a difference of 13.9% (P = 0.026). None of the patients achieving the A1C target experienced an episode of severe hypoglycemia or DKA.
Nonglycemic endpoints
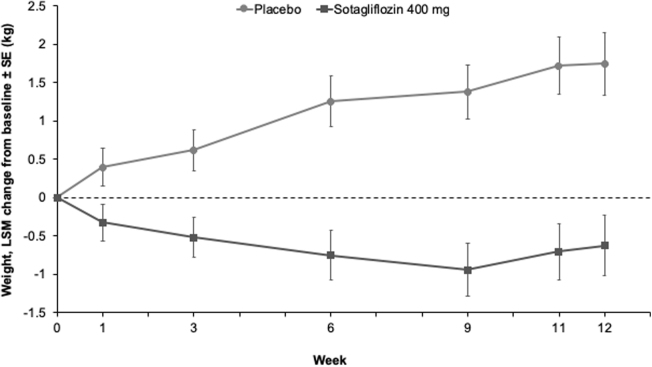
By week 1, sotagliflozin was associated with weight loss compared with placebo (−0.7 kg [95% CI: −1.4 to −0.03; P = 0.041]; Fig. 3), and by week 12, weight had decreased by 2.4 kg (95% CI: −3.5 to −1.2; P < 0.001) with sotagliflozin relative to placebo (Table 2).
FIG. 3.
LSM change from baseline to week 12 in weight in the mITT population.
No significant changes in eGFR were noted in the sotagliflozin group at week 6 (−1.8 ± 3.0 mL/min/1.73 m2; P = 0.55 vs. placebo) and week 12 (−3.3 ± 3.1 mL/min/1.73 m2; P = 0.30) (Table 2). The eGFR returned to baseline 1 week after treatment ended.
Hypoglycemia and DKA
A total of 77 (90.6%) patients had at least 1 documented hypoglycemic event: 38 (90.5%) in the placebo group (75.3 events/patient per year) and 39 (90.7%) in the sotagliflozin group (60.2 events/patient per year). Positively adjudicated severe hypoglycemia was reported by 2 (4.8%) patients receiving placebo (both in the MDI subgroup) and 1 (2.3%) receiving sotagliflozin (CSII subgroup) (Table 3).
Table 3.
Summary of Adverse Events
| Event, n (%) | Placebo (n = 42) | Sotagliflozin 400 mg (n = 43) |
|---|---|---|
| Any AE | 26 (61.9) | 25 (58.1) |
| Drug-related AE | 6 (14.3) | 9 (20.9) |
| Severe drug-related AE | 1 (2.4) | 1 (2.3) |
| Serious AE | 3 (7.1) | 2 (4.7) |
| Drug-related serious AE | 2 (4.8) | 1 (2.3) |
| AEs leading to treatment discontinuation | 2 (4.8) | 0 |
| Drug-related AEs leading to treatment discontinuation | 1 (2.4) | 0 |
| Deaths | 0 | 0 |
| AEs occurring in ≥5% of patients in any group | ||
| Blood ketone body increased | 3 (7.1) | 8 (18.6) |
| Nasopharyngitis | 4 (9.5) | 5 (11.6) |
| Cough | 1 (2.4) | 3 (7.0) |
| Back pain | 0 | 3 (7.0) |
| Vomiting | 4 (9.5) | 2 (4.7) |
| Upper respiratory tract infection | 3 (7.1) | 2 (4.7) |
| Nausea | 3 (7.1) | 1 (2.3) |
| Events of special interest | 5 (11.9) | 7 (16.3) |
| Diarrhea | 0 | 2 (4.7) |
| Urinary tract infection | 1 (2.4) | 3 (7.0) |
| Vulvovaginal mycotic infection | 0 | 2 (4.7) |
| Cystitis | 1 (2.4) | 0 |
| Bone fracture | 1 (2.4) | 0 |
| Glomerular filtration rate decreased | 1 (2.4) | 0 |
| Volume depletion (PT: dizziness) | 1 (2.4) | 0 |
| Pancreatitis | 0 | 0 |
| Malignancy | 0 | 0 |
| Documented hypoglycemia (≤70 mg/dL) | 38 (90.5) | 39 (90.7) |
| Positively adjudicated events of special interest | 3 (7.1) | 1 (2.3) |
| Severe hypoglycemia | 2 (4.8) | 1 (2.3) |
| DKA | 1 (2.4) | 0 |
| Drug-induced liver injury | 0 | 0 |
AE, adverse event; DKA, diabetic ketoacidosis; PT, Preferred Term.
Overall, 13 (15.3%) patients had possible acidosis-related AEs, including 5 (11.9%) in the placebo group and 8 (18.6%) in the sotagliflozin group. Of these, three possible metabolic acidosis events (two with placebo, one with sotagliflozin) were reported. The event occurring in the sotagliflozin group, which occurred in a patient with pneumonia, was not adjudicated as DKA or metabolic acidosis. One of the two events occurring in a placebo-treated patient (MDI subgroup) was adjudicated as DKA but began during the follow-up period and was not considered drug related (Table 3).
Additional safety findings
Overall, 26 (61.9%) patients in the placebo group and 25 (58.1%) patients in the sotagliflozin group experienced an AE during the study (Table 3). There were no deaths, major adverse cardiovascular events, drug-induced liver injuries, amputations, pancreatitis, or malignancies. A femoral neck fracture in a placebo-treated patient was the only reported bone fracture and was considered a serious, non-drug-related AE. Overall, serious AEs occurred in 3 (7.1%) patients receiving placebo and 2 (4.7%) receiving sotagliflozin; of these, two events in the placebo group (investigator-reported [but not adjudicated] DKA and severe hypoglycemia) and one in the sotagliflozin group (severe hypoglycemia) were considered drug related.
Majority of AEs were generally mild to moderate; severe AEs occurred in four patients (severe vomiting and severe hypoglycemia in the placebo group and severe pneumonia and severe hypoglycemia in the sotagliflozin group). Drug-related AEs were reported by 9 (20.9%) sotagliflozin-treated patients and 6 (14.3%) patients receiving placebo. The most common AEs occurring in ≥5% patients and more frequently in the sotagliflozin than the placebo group were increases in blood ketone bodies, nasopharyngitis, urinary tract infection, cough, and back pain. Genital mycotic infections (consistent with SGLT2 inhibition in the kidney) and diarrhea (consistent with partial SGLT1 inhibition in the intestine) were each reported by two patients in the sotagliflozin group and none in the placebo group (Table 3).
Discussion
In this small, 12-week, randomized, double-blind study, large A1C reductions were observed both with sotagliflozin and with placebo added to insulin in young adult patients with poorly controlled T1D, likely reflecting the impact of participation in a randomized trial with frequent, protocol-driven interactions and assessments. The nonstatistically significant between-group treatment difference in A1C of -0.4% favoring sotagliflozin in this study is generally consistent with between-treatment differences that achieved statistical significance in larger phase 3 studies of SGLT inhibitors in adults with T1D.8–13 Sotagliflozin enabled more patients in this study to achieve an A1C of <7% and significantly reduced 2-h PPG and body weight over 12 weeks. In subgroups of patients with a screening A1C ≤10.0% or receiving insulin via CSII, A1C reductions were significantly greater with sotagliflozin than placebo. The incidence of documented and severe hypoglycemia did not differ between treatment groups. No DKA occurred among sotagliflozin-treated patients in this high-risk population over 12 weeks, with one DKA episode in the placebo group.
The placebo response in this study was robust, which is not entirely surprising in a population that, by design, had poor glycemic control at study entry. The study's inclusion criteria were chosen to reflect glycemic control among the general population of young adults with T1D. Younger patients frequently have worse glycemic control than other age groups due to a wide variety of personal, developmental, and societal factors, putting them at high risk of acute and chronic complications of hyperglycemia.1–4,7 The 1.0% decrease in A1C after 12 weeks in the placebo group in the present trial is considerably larger than changes observed in placebo groups in other phase 2 trials of SGLT inhibitors added to insulin therapy in T1D.15,16 This reduction may reflect a higher intensity of disease management for these patients, possibly associated with the high frequency of contact with health care providers that typifies the clinical trial setting.17 Within the MDI subgroup, increases in mean daily basal insulin doses over the treatment period further suggest that the placebo-treated patients may have been attempting to achieve glycemic targets. In contrast, patients on CSII regimens experienced a 0.6% greater A1C decrease with sotagliflozin treatment versus placebo. Reasons for this difference are unclear, but we could speculate that, given the nature of their therapeutic regimens, patients using CSII may not have needed to “step up” their self-care routines as much as patients using MDI to meet the trial's treat-to-target daily glucose goals, and thus these patients saw a greater benefit from supplementation with an active adjunct to insulin treatment.
Younger adults with T1D are also not well studied, particularly in terms of adjunctive therapy with SGLT inhibitors. This study included adults 18–30 years of age, with a mean age of 22 years. Although 18-year-old adults were eligible for inclusion in studies of SGLT2 inhibitors in T1D, all but one trial involved patient populations with a mean age in the mid-40s.8–13,18 The mean age of ∼35 years in a phase 2 study of dapagliflozin was still outside the inclusion criteria of the present trial, and well above the mean age of this study's participants.18
Adolescents and young adults have the highest rates of DKA among patients with T1D. The risk of DKA is increased when SGLT inhibitors are combined with intensive insulin therapy.19 In phase 3 trials conducted to date in patients with T1D, an absolute incidence of DKA of ∼2%–4% has been reported with sotagliflozin, dapagliflozin, and empagliflozin at the doses currently approved for patients with T2D in trials variously lasting 6 months to 1 year.8–13,19,20 Because of this known risk, DKA mitigation was an important component of the sotagliflozin clinical trial program for T1D, and all trials provided education to patients, investigators, and study staff about the prevention of DKA and its early detection and management.8–10,20
In the present trial involving patients at high risk of DKA, there was a higher incidence of elevated blood ketones, but there were no positively adjudicated cases of DKA among sotagliflozin-treated participants, suggesting that DKA mitigation is possible. When SGLT inhibitors are prescribed with intensive insulin therapy, ketones should be monitored in at-risk situations, regardless of the patient's blood glucose level. If a patient is not able to drink or eat, such as before a surgical procedure, due to a gastrointestinal infection or other systemic illness, or any other reason, the patient should stop the SGLT inhibitor until they can resume eating and drinking. The SGLT inhibitor therapy should be discontinued in advance of any planned activities that predispose patients to being unable to eat or that increase their potential for dehydration. Several proposals and consensus statements further describe strategies for the prevention, diagnosis, and management of DKA.21–23
Although this trial fulfills a need for studies involving younger patients with T1D, our conclusions are limited by the small population size and short duration of this phase 2 trial. Because statistical significance was hierarchically tested, and the primary endpoint was not met, results for secondary and other endpoints can only be described descriptively and cannot be used to declare statistical significance. Nevertheless, the limited experience gained from this study provides reassurance for safe use in a high-risk population with appropriate education and close follow-up.
In conclusion, in young adults with poorly controlled T1D, the combination of sotagliflozin with background insulin improved postprandial glycemic control and body weight with a numerical reduction in A1C. Sotagliflozin was generally well tolerated without an increased incidence of DKA and hypoglycemia in this high-risk T1D group.
Supplementary Material
Acknowledgments
The authors thank the JDRF for their co-sponsorship of this trial as well as the investigators, staff, and patients for their participation. They also thank Michael Davies, MD, Lexicon Pharmaceuticals, Inc., The Woodlands, TX, for reviewing the article. Amanda Justice, independent consultant, Brooklyn, NY, provided medical writing and editorial support, which was funded by Lexicon Pharmaceuticals, Inc.
Author Disclosure Statement
B.W.B. has served as a consultant for Adocia, Eli Lilly and Company, Medtronic, Novo Nordisk, Lexicon Pharmaceuticals, Inc., and Pfizer; has served on speaker's bureaus for Astra Zeneca, Lilly/Boehringer Ingelheim, Janssen, Medtronic, Novo Nordisk, Sanofi, and Senseonics; and owns stock in Aseko; and his employer (Atlanta Diabetes Associates) has received grant and/or research support from Abbott, DexCom, Diasome, GSK, Janssen, Insulet, Lexicon Pharmaceuticals, Inc., Lilly/Boehringer Ingelheim, Mannkind, Medtronic, the National Institutes of Health, Nova Biomedical, Novo Nordisk, Provention Bio, Sanofi, Senseonics, REMD Biotherapeutics, Xeris, and vTv Therapeutics LLC. E.C. is a speaker for Novo Nordisk and serves on the advisory board for Novo Nordisk, MannKind, Adocia, Arecor, and Lexicon. R.P.W. has acted as an advisory board member for Eli Lilly and Medtronic, as a speaker and consultant for Dexcom and Tandem Diabetes Care; has received research support from Bigfoot Biomedical, Dexcom, Eli Lilly, MannKind Corporation, Novo Nordisk, and Tandem Diabetes Care and research funding and other support from Lexicon during the conduct of the study. P.B. is employed by and holds stock in Lexicon Pharmaceuticals, Inc. T.D. has acted as consultant, advisory board member, steering committee member, or speaker for Abbott, Medtronic, Roche, Lexicon, Menarini, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Sanofi, Dexcom, and Eli Lilly; has received research grants from Abbott, AstraZeneca, Novo Nordisk, Medtronic, and Sanofi. J.A.K. serves as medical director of McNair Interests, a private equity group with investments in T1D and other chronic illnesses, and he is also an advisor for Sanofi and Lexicon. D.K.M. has received honoraria for clinical trials leadership from GlaxoSmithKline, Janssen, Lexicon, Boehringer Ingelheim, AstraZeneca, Sanofi Aventis, Merck & Co, Pfizer, Novo Nordisk, Esperion, and Lilly USA; consulting fees from AstraZeneca, Merck Sharp & Dohme, GlaxoSmithKline, Lilly USA, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Applied Therapeutics, Metavant, Sanofi Aventis, and Afimmune. A.L.P. has participated on advisory boards for Abbott Diabetes Care, Boehringer Ingelheim, Eli Lilly and Company, MannKind, Medscape, Novo Nordisk, Sanofi, and Lexicon; has received research funding from Dexcom and vTv Therapeutics; and has stock options for Mellitus Health, Pendulum Therapeutics, Omaha Health, Stability Health, and Livongo. P.S. and S.S. were employed by Lexicon Pharmaceuticals at the time the study was conducted. P.S. is now employed by Metavant Sciences, Ltd., and S.S. is employed by Immuvant, Inc.
Funding Information
This study was supported by Lexicon Pharmaceuticals, Inc., and the JDRF through a development agreement and was conducted by Lexicon.
Supplementary Material
References
- 1. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brazeau AS, Nakhla M, Wright M, et al. : Stigma and its association with glycemic control and hypoglycemia in adolescents and young adults with type 1 diabetes: cross-sectional study. J Med Internet Res 2018;20:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas AM, Peterson L, Goldstein D: Problem solving and diabetes regimen adherence by children and adolescents with IDDM in social pressure situations: a reflection of normal development. J Pediatr Psychol 1997;22:541–561 [DOI] [PubMed] [Google Scholar]
- 4. Yi-Frazier JP, Yaptangco M, Semana S, et al. : The association of personal resilience with stress, coping, and diabetes outcomes in adolescents with type 1 diabetes: variable- and person-focused approaches. J Health Psychol 2015;20:1196–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bode BW, Garg SK: The emerging role of adjunctive noninsulin antihyperglycemic therapy in the management of type 1 diabetes. Endocr Pract 2016;22:220–230 [DOI] [PubMed] [Google Scholar]
- 6. Lyons SK, Hermann JM, Miller KM, et al. : Use of adjuvant pharmacotherapy in type 1 diabetes: international comparison of 49,996 individuals in the Prospective Diabetes Follow-up and T1D Exchange Registries. Diabetes Care 2017;40:e139–e140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 8. Garg SK, Henry RR, Banks P, et al. : Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017;377:2337–2348 [DOI] [PubMed] [Google Scholar]
- 9. Buse JB, Garg SK, Rosenstock J, et al. : Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: The North American inTandem1 Study. Diabetes Care 2018;41:1970–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danne T, Cariou B, Banks P, et al. : HbA1c and hypoglycemia reduction at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care 2018;41:1981–1990 [DOI] [PubMed] [Google Scholar]
- 11. Dandona P, Mathieu C, Phillip M, et al. : Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:864–876 [DOI] [PubMed] [Google Scholar]
- 12. Mathieu C, Dandona P, Gillard P, et al. : Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 Study): 24-week results from a randomized controlled trial. Diabetes Care 2018;41:1938–1946 [DOI] [PubMed] [Google Scholar]
- 13. Rosenstock J, Marquard J, Laffel LM, et al. : Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018;41:2560–2569 [DOI] [PubMed] [Google Scholar]
- 14. Sands AT, Zambrowicz BP, Rosenstock J, et al. : Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care 2015;38:1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker C, Wason S, Banks P, et al. : Dose-dependent glycometabolic effects of sotagliflozin on type 1 diabetes over 12 weeks: The inTandem4 trial. Diabetes Obes Metab 2019;21:2440–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henry RR, Thakkar P, Tong C, et al. : Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258–2265 [DOI] [PubMed] [Google Scholar]
- 17. Khan A, Fahl Mar K, Schilling J, Brown WA: Magnitude and pattern of placebo response in clinical trials of oral antihyperglycemic agents: data from the U.S. Food and Drug Administration, 1999–2015. Diabetes Care 2018;41:994–1000 [DOI] [PubMed] [Google Scholar]
- 18. Henry RR, Rosenstock J, Edelman S, et al. : Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care 2015;38:412–419 [DOI] [PubMed] [Google Scholar]
- 19. Peters AL, Buschur EO, Buse JB, et al. : Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garg SK, Strumph P: Effects of sotagliflozin added to insulin in type 1 diabetes. N Engl J Med 2018;378:967–968 [DOI] [PubMed] [Google Scholar]
- 21. Danne T, Garg S, Peters AL, et al. : International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 2019; DOI: 10.2337/dc18-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garg SK, Peters AL, Buse JB, Danne T: Strategy for Mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther 2018;20:571–575 [DOI] [PubMed] [Google Scholar]
- 23. American Diabetes Association: 15. Diabetes care in the hospital: standards of medical care in diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S173–S181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.