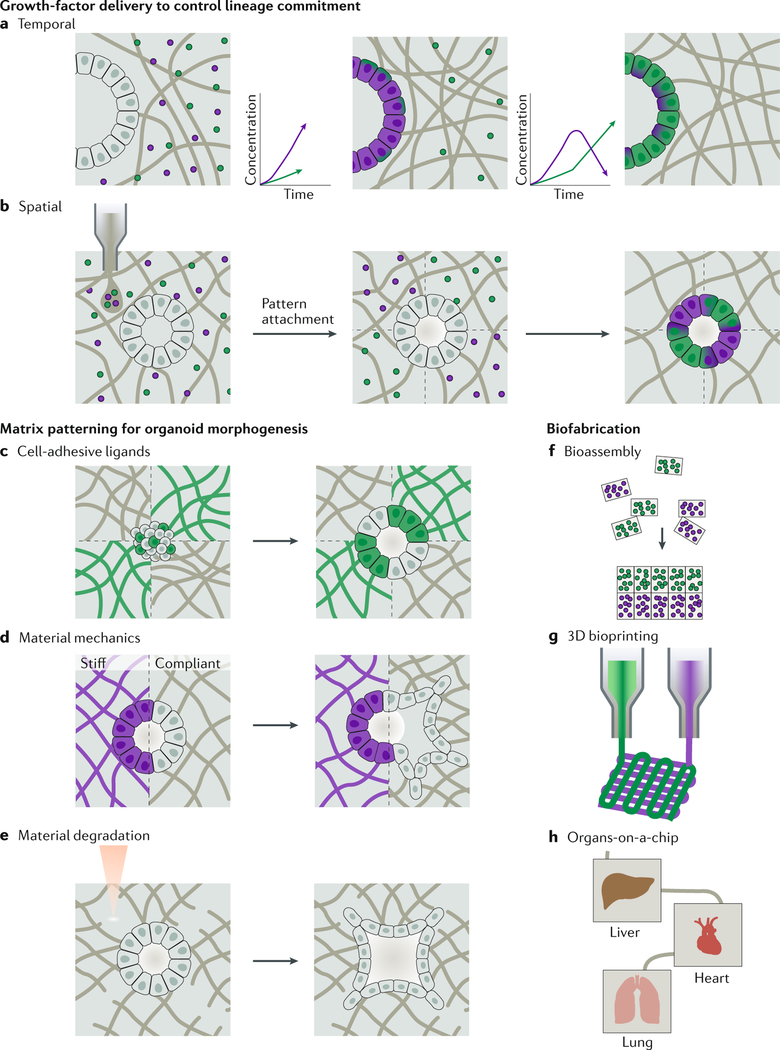
Fig. 4 |. Dynamic organoid niches.
Engineered materials may enable the control of lineage commitment of stem and progenitor cells in time and space. a | The release profile of matrix-immobilized growth factors can be designed to sequentially deliver signals through growth factor-material affinity or proteolytic release, providing the appropriate biochemical cues for maturing organoids. b | Materials can be patterned with growth factors to spatially control lineage commitment and, thus, the development and maturation of organoids. c | Materials can be patterned with cell-type-specific adhesive ligands to guide cell self-sorting of early cell clusters and organoids. d | The mechanical properties of a material impact cellular migration. e | Material degradation can be designed to control cellular migration and morphogenesis of the developing organoid. f | Biofabrication techniques provide opportunities to produce spatially controlled, engineered matrices for organoid culture. Bioassembly could be used to spontaneously form microtissues with zonal organization of region-specific organoids. g | Bioprinting enables the rapid fabrication of complex tissue architectures, for example, vascularized constructs. h | Organoids can also be incorporated into organ-on-a-chip platforms. These platforms can provide powerful models of clinically relevant multi-organ interactions256–270.