Abstract
MicroRNA-30a (miR-30a) impacts adipocyte function, and its expression in white adipose tissue (WAT) correlates with insulin sensitivity in obesity. Bioinformatic analysis demonstrates that miR-30a expression contributes to 2% of all miRNA expression in human tissues. However, molecular mechanisms of miR-30a function in fat cells remain unclear. Here, we expanded our understanding of how miR-30a expression contributes to antidiabetic peroxisome proliferator-activated receptor-γ (PPARγ) agonist activity and metabolic functions in adipocytes. We found that WAT isolated from diabetic patients shows reduced miR-30a levels and diminished expression of the canonical PPARγ target genes ADIPOQ and FABP4 relative to lean counterparts. In human adipocytes, miR-30a required PPARγ for maximal expression, and the PPARγ agonist rosiglitazone robustly induced miR-30a but not other miR-30 family members. Transcriptional activity studies in human adipocytes also revealed that ectopic expression of miR-30a enhanced the activity of rosiglitazone coupled with higher expression of fatty acid and glucose metabolism markers. Diabetic mice that overexpress ectopic miR-30a in subcutaneous WAT display durable reductions in serum glucose and insulin levels for more than 30 days. In agreement with our in vitro findings, RNA-seq coupled with Gene Set Enrichment Analysis (GSEA) suggested that miR-30a enabled activation of the beige fat program in vivo, as evidenced by enhanced mitochondrial biogenesis and induction of UCP1 expression. Metabolomic and gene expression profiling established that the long-term effects of ectopic miR-30a expression enable accelerated glucose metabolism coupled with subcutaneous WAT hyperplasia. Together, we establish a putative role of miR-30a in mediating PPARγ activity and advancing metabolic programs of white to beige fat conversion.
Keywords: metabolism, microRNA, mitochondria, PPARγ, subcutaneous adipocytes
INTRODUCTION
White adipose tissue (WAT) critically mediates the organismal response to energy demands and nutrient availability. Increased adipocyte size (hypertrophy) is a hallmark of WAT enlargement in obesity. It is typically associated with deleterious metabolic alterations, proinflammatory response, and increased risk of developing type 2 diabetes mellitus (T2DM) independent of total fat mass (58). T2DM is distinctly associated with defective adipocyte function, particularly disruption of their capacity to store energy as lipids and secrete hormones that regulate systemic insulin sensitivity (53).
Subcutaneous WAT represents the largest adipose tissue depot in humans and safely stores surfeit energy for metabolic demands. Recruitment of new adipocytes through hyperplasia in subcutaneous WAT, when needed to store excess energy, enables healthy adipose tissue expansion and protects against metabolic disease (23). Subcutaneous WAT hyperplasia (more, smaller fat cells) has been linked to increased gene expression of transcriptional regulators essential for adipocyte differentiation, such as peroxisome proliferator-activated receptor-γ (PPARγ) (59). However, persistent states of positive energy balance support hypertrophic expansion of subcutaneous adipocytes (bigger fat cell size) coupled with increased inflammation and insulin resistance (23). Although the molecular mechanisms underpinning these observations remain incompletely understood, subcutaneous WAT safely sequesters excess energy and exerts metabolically protective effects in obesity.
PPARγ regulates adipocyte differentiation, and its activation exerts therapeutic effects that ultimately improve metabolic profiles in T2DM (54). Mechanistically, ligands activate PPARγ by stabilizing the ligand-binding domain in an active conformation (9, 44) to govern transcription of genes involved in glucose and lipid homeostasis. Thiazolidinediones (TZDs) such as rosiglitazone (Avandia) and pioglitazone (Actos) act as high-affinity PPARγ agonists to enhance insulin sensitivity and reduce fasting blood glucose levels (15, 19, 32–34). TZDs also promote insulin sensitivity by directing fatty acids to subcutaneous WAT rather than central WAT depots (32). Interestingly, subcutaneous white fat cells exposed to TZDs show a capability to acquire some brown fat features, including the expression of uncoupling protein 1 (UCP1) and increased mitochondrial biogenesis (7, 45, 46, 61). These changes confer metabolically beneficial effects for the organism and, ultimately, reflect white-to-brown fat conversion to the phenotype termed “beige” cells. However, TZDs have many perceived toxicities (54). Similarly to TZDs, PPARγ partial agonists improve insulin sensitivity and reduce fat deposition in all depots but do not exert uniform benefits (45). Clearly, selective PPARγ modulation to increase insulin sensitivity without undesirable side effects is warranted. One other therapeutic strategy would be to identify and leverage downstream targets of PPARγ that engender TZD effects.
MicroRNAs (miRNAs) are noncoding RNAs of 20–25 nucleotides that bind target mRNAs in the 3′-untranslated region (UTR) to induce mRNA degradation and inhibit translation (5). Several miRNAs correlate with human obesity and T2DM, and multiple studies now demonstrate that miRNAs influence metabolism by regulating adipocyte differentiation, inflammation, and metabolic functions (25). The most abundant miRNAs target hundreds of genes and regulate overlapping signaling pathways. However, miRNAs frequently exert relatively modest effects on individual mRNA targets, acting as rheostats to finely regulate protein expression (25). Given the large number of miRNAs, miRNAs govern critical aspects of energy metabolism, glucose homeostasis, and whole body insulin sensitivity.
We recently established that the miRNA miR-30a improves insulin sensitivity in obesity (35, 36). But primary mechanisms of miR-30a regulation and functional outcomes remain elusive. miR-30a expression is reduced in WAT isolated from mice and humans exhibiting insulin resistance, which also motivates new studies to establish how miR-30a promotes fatty acid and glucose metabolism in adipocytes. The current study outlines a mechanism coupling PPARγ activity with durable metabolic impacts of miR-30a in subcutaneous WAT. Overall, our results reveal a critical role of miR-30a in activating PPARγ to drive white-to-beige fat conversion and subcutaneous WAT expansion.
MATERIALS AND METHODS
Human subjects.
Subcutaneous adipose tissue biopsies (∼500 mg) from the lateral thigh were obtained from lean volunteers (BMI = 23.9 ± 0.5, fasting plasma glucose = 80.3 ± 3 mg/dL, HOMA-IR = 1.0 ± 0.11), obese subjects (BMI = 38.3 ± 1.5, fasting plasma glucose = 82 ± 3 mg/dl, HOMA-IR = 2.2 ± 0.3), and patients with recently diagnosed T2DM (BMI = 35.2 ± 3.8, fasting plasma glucose = 126 ± 31 mg/dl, HOMA-IR = 7.8 ± 2.1) and used for quantitative PCR (qPCR) analysis (24). These studies were approved by the Baylor College of Medicine (BCM) Institutional Review Board. All participants provided written, informed consent.
Cell culture.
Cryopreserved subcutaneous primary human preadipocytes from normal female donors with a mean BMI of 27.51 were provided by Zen-Bio, Inc. (Research Triangle Park, NC). Cells were received at passage 2 and experiments performed before cells reached passage 10. Experiments were performed using pooled human preadipocytes from five individual female donors (lot SL0065). Human preadipocytes were maintained in DMEM-F-12 with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin (growth media). Confluent cells were differentiated using growth media supplemented with 100 nM human insulin, 0.250 mM 3-isobutyl-1-methylxanthine (IBMX), 500 nM dexamethasone, and 3 μM rosiglitazone. Based on previous microarray analysis and other studies (26, 27), adipocytes were considered mature on day 8. After differentiation, we treated adipocytes with 1 μM rosiglitazone in low-serum OPTI-MEM media formulations (ThermoFisher) for ≤4 days.
miRNA and siRNA transfection.
Mature adipocytes were transfected with PPARG siRNA (Qiagen), mismatch siRNA control (Qiagen), miR-30a-5p mimic (Active Motif), or control mimic (Active Motif) at a final concentration of 20 nM using Dharmafect transfection reagent (Dharmacon). After transfection, cells were incubated for 48 h at 5% CO2/37°C. We performed transfection and rosiglitazone treatment studies in low-serum OPTI-MEM media formulations.
RNA extraction, mitochondrial DNA analysis, and qPCR.
Total RNA was extracted from cells using the miRNeasy kit (Qiagen). cDNA was synthesized using SuperScript ViLO Master Mix (ThermoFisher) from 200 ng of total RNA. To measure relative mRNA expression, qPCR was performed with TaqMan reagents using a StepOne real-time PCR system (Applied Biosystems). Invariant controls included 18S rRNA and TBP. TaqMan Gene Expression and Roche Universal Probe Library assays (Table 1) were used as previously described (35, 36).
Table 1.
TaqMan assays used in quantitative RT-PCR
| Gene | Species | TaqMan Assay |
|---|---|---|
| PPARG | Human | Hs01115513_m1 |
| FABP4 | Human | Hs01086177_m1 |
| PPARGC1A | Human | Hs01016719_m1 |
| CIDEA | Human | Hs00154455_m1 |
CIDEA, cell death-inducing DNA fragmentation factor-α (DFFA)-like effector A; FABP4, fatty acid-binding protein 4; PPARG, peroxisome proliferator-activated receptor-γ; PPARGC1A, PPARG coactivator 1α.
The TaqMan Advanced miRNA cDNA Synthesis Kit (no. A28007; ThermoFisher) was used to synthesize miRNA cDNA from 20 ng of total RNA. To extend mature miRNAs, polyadenylation and adaptor sequence ligation of the 3′ and 5′ ends, respectively, occurs before universal priming and reverse transcription. To address low-expressing targets, cDNA is amplified by primers that recognize sequences appended to both ends, effectively minimizing amplification bias (Table 2). Next, the TaqMan Advanced miRNA Assays (no. A25576; ThermoFisher) were used to quantify relative gene expression. The invariant ncRNA control was RNU48 (human) or sno412 (mouse).
Table 2.
Roche Universal Probe Library primer sequences used in quantitative RT-PCR for markers of adipocyte differentiation and thermogenesis in fat cells and tissues
| Gene | Species | Accession No. | L Primer | R Primer | UPL Probe No. |
|---|---|---|---|---|---|
| ADIPOQ | Human | NM_004797.2 | ggtgagaagggtgagaaagga | tttcaccgatgtctcccttag | 85 |
| LPL | Human | NM_000237.2 | atgtggcccggtttatca | ctgtatcccaagagatggacatt | 25 |
| DIO2 | Human | NM_013989.3 | ggaagagcttcctcctcgat | tccttctgtactggagacatgc | 47 |
| TBP | Human | NM_003194.4 | cccatgactcccatgacc | tttacaaccaagattcactgtgg | 51 |
| 18S | Human | ctcaacacgggaaacctcac | cgctccaccaactaagaacg | 77 | |
| Pparg | Mouse | NM_011146.3 | gaaagacaacggacaaatcacc | gggggtgatatgtttgaacttg | 7 |
| Adipoq | Mouse | NM_009605.4 | ggagagaaaggagatgcaggt | ctttcctgccaggggttc | 17 |
| Fabp4 | Mouse | NM_024406.2 | gggatggaaagtcgaccacaa | tggaagtcacgcctttcata | 31 |
| Ppargc1a | Mouse | NM_008904.2 | gaaagggccaaacagagaga | gtaaatcacacggcgctctt | 29 |
| Tbp | Mouse | NM_013684.3 | cggtcgcgtcattttctc | gggttatcttcacacaccatga | 107 |
| Cidea | Mouse | NM_007702.2 | aaaccatgaccgaagtagcc | aggccagttgtgatgactaagac | 66 |
| Ucp1 | Mouse | NM_009463.3 | ggcctctacgactcagtcca | taagccggctgagatcttgt | 34 |
| Cd36 | Mouse | NM_001159558.1 | ttgtacctatactgtggctaaatgaga | cttgtgttttgaacatttctgctt | 9 |
| Dio2 | Mouse | NM_010050.2 | ctgcgctgtgtctggaac | ggagcatcttcacccagttt | 69 |
ADIPOQ, adiponectin; Cidea, cell death-inducing DNA fragmentation factor-α (DFFA)-like effector A; DIO2, iodothyronine deiodinase 2; Fabp4, fatty acid-binding protein 4; LPL, lipoprotein lipase; Pparg, peroxisome proliferator-activated receptor-γ; Ppargc1a, PPARG coactivator 1α; TBP, TATA-binding protein; Ucp1, uncoupling protein 1.
Mitochondrial DNA content was determined by qPCR. Total DNA was isolated using the DNeasy kit (Qiagen). Real-time qPCR was performed on the StepOne real-time qPCR system (Applied Biosystems) using Platinum SYBR Green qPCR Supermix-UDG (Life Technologies). Reactions were prepared according to the manufacturer’s recommendations in a total volume of 25 µL. mtDNA primers were designed using the human mitochondrial genome sequence within the NADH dehydrogenase subunit 6 (ND6) gene. 18S rRNA was used as the invariant control.
Luciferase reporter assays.
We used replication-deficient adenoviruses coupled with poly-lysine (2) to express PPRE-luc luciferase fusions in human adipocytes. Reporter gene activity was detected using the Promega Luciferase Assay Kit. Relative luminescence units were normalized to β-galactosidase activity (Sigma).
Antibodies.
The following antibodies were used for immunoblotting: PPARγ (no. 2435; Cell Signaling Technology), HSP90 (no. 4877; Cell Signaling Technology), UCP1 (no. ab10983; Abcam), ADIPOQ (no. GTX112777; Genetex), CD36 (no. sc-9154; Santa Cruz Biotechnology), CPT1 (no. sc-98834; Santa Cruz Biotechnology), and FABP4 (no. GTX116036; Genetex).
Immunoblotting.
Cells were collected by scraping and lysed in RIPA buffer supplemented with the appropriate protease and phosphatase inhibitors. Immunoblot analysis was performed with whole cell lysates run on 4–12% Bis-Tris NuPage (Millipore) gels and transferred onto Immobilon-P Transfer Membranes (Millipore), followed by antibody incubation. Immunoreactive bands were visualized by chemiluminescence.
Animal care and use.
All procedures involving animals were approved by the Baylor College of Medicine Institutional Animal Care Committee (protocol no. AN-6411). Experimental animals received humane care according to criteria in the Guide for the Care and Use of Laboratory Animals (8th ed., revised 2011). Six-week-old male C57BL/6J wild-type mice (no. 000664) were purchased from The Jackson laboratory and fed a 60% high-fat diet (HFD) (no. D12492; Research Diets) for 14 wk before experiments. All experimental animals were housed (no more than 4 per cage) in a barrier-specific pathogen-free animal facility with 12-h dark-light cycle and free access to water and food. Mouse body composition was examined by MRI (Echo Medical Systems). We obtained Adv-miR-control (m009) and Adv-miR-30a (mm0332) from Applied Biological Materials, Inc. Adv (5 × 109 pfu/ml) was injected into both left and right inguinal fat pads of male mice (HFD 14 wk) under anesthesia. Mice were anesthetized with isoflurane (5%) and maintained using 2% isoflurane for injections. At the end of the experiments, mice were euthanized by cervical dislocation, which was performed under isoflurane anesthesia. After euthanasia, tissues were collected, flash-frozen in liquid N2, and stored at −80°C until use. All experiments adhered to the ARRIVE guidelines.
Hormone, glucose, and metabolite profiles.
Four-hour-fasted plasma insulin levels (EZRMI-13K; EMD Millipore) and adiponectin (KMP0041; ThermoFisher) were quantified using ELISA. Blood glucose was measured with a Freestyle Glucose Monitoring System (Abbott Laboratories) during routine monitoring of metabolic parameters.
Histology.
For immunohistochemistry of WAT, paraffin-embedded sections were stained with hematoxylin and eosin (H & E) and imaged using a Nikon Ci-L Bright-field microscope. Adiposoft coupled with Fiji software quantified adipocyte morphometry in histological sections of WAT (18).
Analysis of RNA-seq data.
Poly-A RNA was purified from total RNA using Dynabeads Oligo dT25 (Invitrogen) and fragmented for size selection. First-strand cDNA was synthesized using Superscript Reverse Transcriptase III (Invitrogen). Second strand cDNA was synthesized and marked with dUTP. The resultant cDNA was used for end repair, A-tailing, and adaptor ligation. The second-strand cDNA was then degraded by Uracil-DNA Glycosylase (NEB), and the library was amplified for sequencing (Illumina HiSeq2000). Read pairs were mapped using TopHat2 onto the UCSC mouse genome (mm10) and the RefSeq compendium of genes. Gene expression was computed using Cufflinks2. The RNA-Seq data set can be accessed at the Gene Expression Omnibus (GSE39342).
GSEA was carried out using the GSEA software package (http://software.broadinstitute.org/gsea/index.jsp) to assess the degree of similarity among Adv-miR-30a treatments and known mouse beige, brown, and white adipocyte gene signatures (62) accessed from GSE39562. Normalized Enrichment Score (NES) and adjusted q values (q < 0.25) were computed utilizing the GSEA method based on 1,000 random permutations of the ranked genes.
Metabolomics.
Frozen tissue (100 mg) isolated 35 days after Adv injection (n = 5/treatment group) was submitted to the Metabolomics Core at BCM for targeted metabolite analysis. For extraction of inguinal WAT (iWAT) metabolites, 750 μL of water-methanol (1:4) was added to 100 mg snap-frozen iWAT, and samples were homogenized and then mixed with 450 μL of ice-cold chloroform. The resulting solution was mixed with 150 μL of ice-cold water and vortexed again for 2 min. The solution was incubated at –20°C for 20 min and centrifuged at 4°C for 10 min to partition the aqueous and organic layers. The aqueous and organic layers were combined and dried at 37°C for 45 min in an automatic Environmental Speed-Vac system (Thermo Fisher Scientific). The extract was reconstituted in a 500-μL solution of ice-cold methanol-water (1:1) and filtered through a 3-kDa molecular filter (Amicon Ultracel 3-kDa Membrane) at 4°C for 90 min to remove proteins. The filtrate was dried at 37°C for 45 min in a speed vacuum and stored at –80°C.
Aqueous phase chromatographic separation was achieved using three solvents: water (solvent A), water with 5 mM ammonium acetate (pH 9.9), and 100% acetonitrile (solvent B). The binary pump flow rate was 0.2 mL/min with a gradient spanning 80% B to 2% B over a 20-min period, followed by 2% B to 80% B for a 5-min period and followed by 80% B for a 13-min time period. The flow rate was gradually increased during the separation from 0.2 mL/min (0–20 min), 0.3 mL/min (20–25 min), 0.35 mL/min (25–30 min), and 0.4 mL/min (30–37.99 min) and finally set at 0.2 mL/min (5 min). Glycolytic and TCA cycle intermediates were separated on a Luna Amino (NH2) column (3 µm, 100A 2 × 150 mm; Phenomenex) that was maintained in a temperature-controlled chamber (37°C). Glycolytic and TCA cycle intermediates were measured using negative ionization mode with an ESI voltage of −3,500 ev. Approximately nine to 12 data points were acquired per detected metabolite. For all samples, 10 microliters of sample was injected and analyzed using a 6495 QQQ triple quadrupole mass spectrometer (Agilent) coupled to a 1290 series HPLC system via single-reaction monitoring fragmentation. The data were acquired using mass hunter software and analyzed using mass hunter quantitative analysis software (Agilent). The data were log2-transformed and normalized with internal standards on a per-sample basis. For every metabolite in the normalized data set, unpaired t tests were conducted to compare expression levels between different groups. Differential metabolites were identified by adjusting the P values for multiple testing at a false discovery rate (FDR) threshold of <0.25.
Statistics.
Data presented were acquired from a minimum of three independent experiments performed on multiple days, unless otherwise indicated. We utilized GraphPad Prism 8.4.2 (GraphPad Software) for graphs and statistical analysis. For comparisons between two independent groups, an unpaired, two-tailed t test was used. One-way ANOVA followed by Tukey post hoc tests was used to compare more than two independent groups. Differences between transfections and ligand treatments were determined by two-way ANOVA, followed by Tukey post hoc tests. All data are normally distributed as determined by Shapiro-Wilk and Anderson-Darling tests at α = 0.05. Our primary threshold for statistical significance was P < 0.05.
RESULTS
miR-30a is abundantly expressed across human tissues.
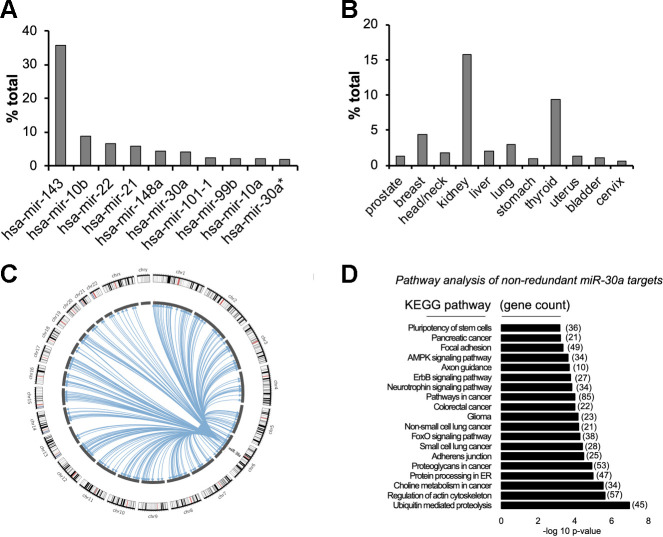
The TCGA Pan-Cancer data set provides the most extensive compilation of miRNA sequencing data in normal tissues produced to date (21). Despite the annotation of more than 2,000 miRNAs, global analysis of miRNA expression patterns in 334 normal tissue samples revealed that the top 10 miRNAs comprise ∼75% of all miRNA expression across normal tissues. miR-30a constitutes 4.1% of total miRNA expression (Fig. 1A). Of the normal tissues available in the TCGA, miR-30a levels vary between 15% (kidney) and 1.6% (cervix) of the total miRNA pool (Fig. 1B).
Fig. 1.
Broad expression of miR-30a in normal tissues. A and B: The Cancer Genome Atlas (TCGA) miRNA expression across normal tissues demonstrated expression of the 10 most abundant miRNAs (A) and fractional expression of miR-30a-5p (B). *miR-30a-3p strand expression. C: bioinformatic analysis of argonaute cross-linking immunoprecipitation (AGO-CLIP) experiments showed that miR-30a binds to many 3′-untranslated region (3′-UTR) targets across the genome. D: Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis broadly categorized miR-30a targets into growth pathways. AMPK, AMP-activated protein kinase.
Argonaute Crosslinking Immunoprecipitation (AGO-CLIP) data sets experimentally identify miRNA-target interactions in a genome-wide manner through purification of Argonaute-protein-associated RNAs, which include bound miRNAs and their respective targets (10). We recently leveraged AGO-CLIP data sets and available miRNA prediction algorithms (1, 37) to compile an extensive atlas of human miRNA binding sites (21, 22). Circos plots allowed illustration of 520 most frequently found bona fide miR-30a binding interactions with 3′-UTRs across the genome (Fig. 1C). KEGG analysis (Fig. 1D) of 2,487 cumulative, nonredundant miR-30a targets (1, 37) identified multiple pathways that enable motility (regulation of actin cytoskeleton) and growth (multiple cancer pathways). Our extensive profiling experiments suggest that abundant miR-30a expression targets pathways that oppose maintenance of differentiated cell states.
Insulin sensitivity correlates with miR-30a expression in human WAT.
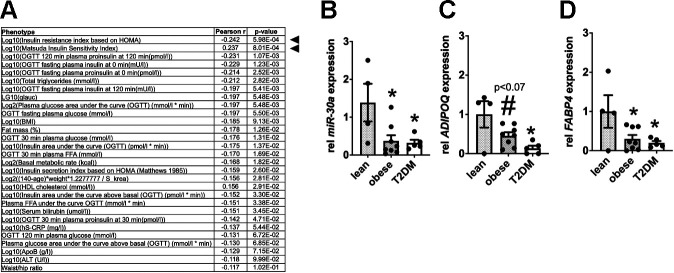
The METSIM study investigated nongenetic and genetic factors associated with the risk of T2DM and cardiovascular disease. Men enrolled in the METSIM study were phenotyped for multiple metabolic features of insulin sensitivity. Small-RNA sequencing of RNA isolated from subcutaneous adipose tissue of 200 donors detected hundreds of miRNAs (12). However, 94% of the miRNAs represented <1% of the total reads. Consistent with our analysis of the TCGA (21, 22), the METSIM study demonstrated that the 15 most abundant miRNAs comprise ∼80% of all sequencing reads. miR-30a-5p accounted for >2% of the total miRNA reads. miR-30a-5p expression strongly correlated with more than 20 metabolic phenotypes (Fig. 2A), including strong negative correlations with HOMA-IR, serum triglycerides, and glucose intolerance [e.g., oral glucose tolerance test (OGTT) measurements]. Furthermore, miR-30a-5p positively correlated with the Matsuda Insulin Sensitivity Index (40), which reflects hepatic and peripheral tissue insulin sensitivity.
Fig. 2.
Obesity and type 2 diabetes mellitus (T2DM) deplete miR-30a levels in human white adipose tissue (WAT). A: correlation of miR-30a with markers of insulin resistance in humans from the Metabolic Syndrome in Men Study (METSIM). Quantitative PCR (qPCR) was used to determine relative miR-30a (B), ADIPOQ (C), and FABP4 (D) expression in subcutaneous adipose tissue biopsied from lean (n = 4), obese (n = 8), and obese type 2 diabetic (n = 5) subjects. Data are represented as means ± SE. *P < 0.05 relative to lean subjects; #P < 0.10 relative to lean subjects determined by 1-way ANOVA and Tukey post hoc tests. Expression levels were normalized to RNU48 or TBP.
Reduced expression of mature adipocyte marker genes corresponds with a predisposition for T2DM and hypertrophic obesity (23). To confirm that changes in miR-30a expression correlate with insulin sensitivity and mature human adipocyte markers, we measured miR-30a levels in subcutaneous adipose tissue isolated from lean, obese, and diabetic subjects. In agreement with the METSIM data (12), we demonstrated that miR-30a levels were significantly lower in obese and insulin-resistant T2DM human subjects compared with insulin-sensitive counterparts (Fig. 2B). We used ADIPOQ (Fig. 2C) and FABP4 (Fig. 2D) expression to confirm alterations in mature adipocyte genes. Together, these results provide evidence that decreased miR-30a expression correlates with reduced levels of mature adipocyte genes in the context of human insulin resistance.
PPARγ regulates miR-30a expression.
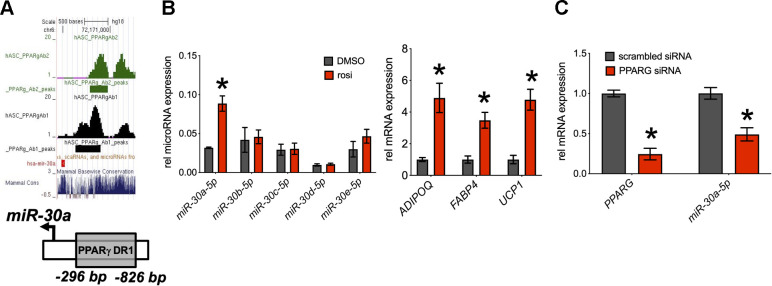
miRNA expression is transcriptionally regulated by conventional transcription factor machinery, including nuclear receptors (14). To date, few miRNAs have been identified as direct PPARγ targets, but our review of multiple genome-wide studies (31, 41, 55) predicts that miR-30a is a conserved, direct PPARγ target. We also performed in silico analysis (Genomatix) of the miR-30a promoter (−1 kb) to identify potential transcription factor binding sites. In agreement with the genome-wide binding studies, we found a PPARγ binding site −286 bp from the miR-30a transcriptional start site (Fig. 3A). To determine whether PPARγ activation affected miR-30a expression, we treated human adipocytes with rosiglitazone. Rosiglitazone sharply increased the expression of miR-30a and the canonical PPARγ target genes ADIPOQ, UCP1, and FABP4 (Fig. 3B). Other miR-30 family members did not respond to rosiglitazone. To further establish potential PPARγ regulation of miR-30a, we decreased PPARG expression by siRNA in human adipocytes (Fig. 3C). After 48 h, we measured the expression of PPARG and miR-30a. Decreasing PPARG expression by 76% resulted in decreased levels of miR-30a (−50%). These results implied that miR-30a is a direct PPARγ target gene.
Fig. 3.
Regulation of miR-30a by PPARγ. A: a proliferator-activated receptor-γ (PPARγ) binding site is in the promoter region of the miR-30a gene, as detected by 2 different antibodies (Ab1 and Ab2). The binding site is highly conserved among mammals (Mammal Cons). B: human adipocytes were treated with rosiglitazone for 4 days. Expression levels of miR-30a, miR-30b, miR-30c, miR-30d, miR-30e, fatty acid-binding protein 4 (FABP4), adiponectin (ADIPOQ), and uncoupling protein 1 (UCP1) were determined by quantitative PCR (qPCR) (n = 3 ± SE). C: mature adipocytes were transfected with PPARγ siRNA or scrambled control. qPCR was used to measure levels of PPARγ and miR-30a (n = 7 ± SE). Expression levels were normalized to TBP or RNU48. Statistical significance between DMSO and rosiglitazone for each molecule was calculated using unpaired, 2-tailed Student’s t test with Sidak-Bonferroni correction for multiple comparisons. *P < 0.05.
miR-30a expression amplifies thiazolidinedione activity in human adipocytes.
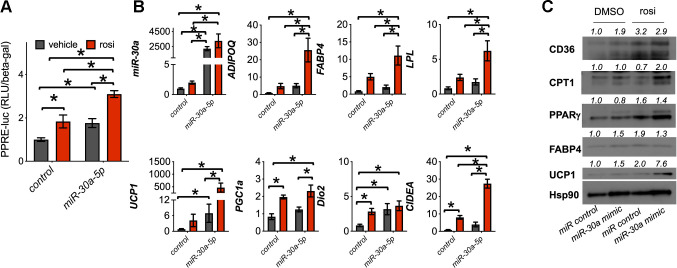
Rosiglitazone transactivates PPARγ to regulate genes in adipocytes necessary for cellular and systemic insulin sensitivity. The induction of miR-30a by PPARγ coincided with transcription of mature white fat genes (Fig. 3). These results suggest that miR-30a activates adipocyte differentiation pathways by amplifying PPARγ activity. To test this notion, we expressed a PPARγ response element (PPRE)-luciferase reporter plasmid in differentiated human adipocytes transfected with miR-30a mimics or a nontargeting miRNA control. miR-30a expression increased both basal and rosiglitazone-induced PPRE-luciferase activity (Fig. 4A), refining our initial observations that implicated miR-30a activates PPARγ function in human adipocytes (35, 36). Another key feature of TZD action is to regulate some brown adipose tissue (BAT) genes that expand the mitochondrial complement necessary for fatty acid metabolic demands (7, 45, 46, 61). Consistent with these reports, miR-30a overexpression induced expression of BAT genes in addition to WAT genes compared with nontargeting miRNA control (Fig. 4B). We also observed that ectopic miR-30a expression enhanced the activity of rosiglitazone, including ligand-dependent elevation of both WAT and BAT PPARγ target genes. Subsequent immunoblot analysis confirmed that miR-30a mimic increased adipocyte marker and mitochondrial lipid metabolism protein levels in a manner similar to rosiglitazone and further enhanced by the combination of miR-30a mimic plus rosiglitazone (Fig. 4C). Together, our findings demonstrate that miR-30a stimulates PPARγ activity.
Fig. 4.
miR-30a stimulates proliferator-activated receptor-γ (PPARγ) transactivation in human adipocytes. To validate effects on PPARγ-mediated transcription, human adipocytes were transfected with miR-30a or control mimics, followed by 1 μM rosiglitazone (rosi) treatment for 4 days. A: transcriptional activity was determined by measuring PPAR response element (PPRE)-luc normalized to β-galactosidase (n = 6 independent experiments ± SE; *P < 0.05). Statistical significance was determined by 2-way ANOVA and Tukey post hoc tests. B: the effects of miR-30a overexpression in human adipocytes were characterized by quantitative PCR (qPCR) analysis of adiponectin (ADIPOQ), fatty acid-binding protein 4 (FABP4), lipoprotein lipase (LPL), uncoupling protein 1 (UCP1), PPARγ coactivator-1α (PGC1a), iodothyronine deiodinase 2 (Dio2), cell death-inducing DNA fragmentation factor-α (DFFA)-like effector A (CIDEA), and miR-30a-5p (n = 6 independent experiments; *P < 0.05 reflects statistical significance between pairs of measurements). Statistical significance was determined by 2-way ANOVA and Tukey post hoc tests. C: expression levels of CD36, carnitine palmitoyl transferase 1 (CPT1), PPARγ, FABP4, and UCP1 were analyzed by immunoblotting. Human adipocytes were transfected with miR-30a mimics and treated with DMSO [vehicle (veh)] or 1 μM rosiglitazone (rosi) for 4 days. Heat shock protein 90 (HSP90) served as the invariant control.
miR-30a expression impacts subcutaneous WAT hyperplasia.
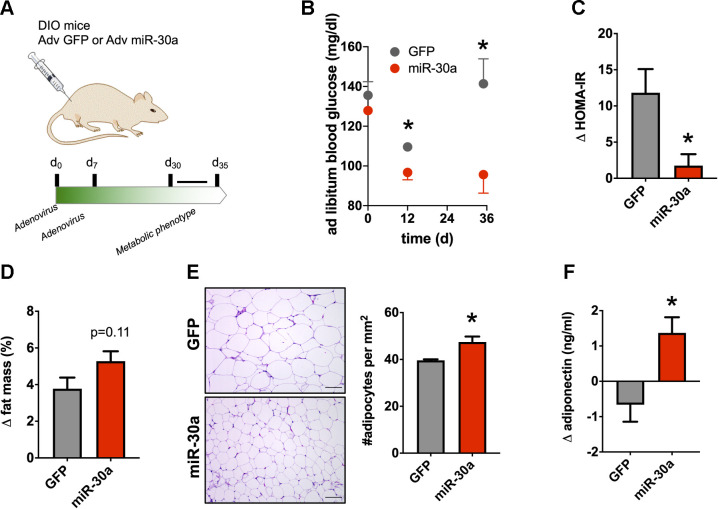
We implemented a method to measure in vivo effects of miR-30a overexpression in subcutaneous (inguinal) murine WAT (16, 47, 60). To expand recent experiments (36) and determine whether miR-30a expression in inguinal WAT caused therapeutic effects in obese mice, we injected the inguinal fat pad of diet-induced obese (DIO) mice twice with an adenovirus (Adv) expressing either miR-30a or GFP (Fig. 5A). One month after injection, body weight for control mice (42.7 ± 1.8 g) remained the same as the Adv-miR-30a-infected group (40.8 ± 2.3 g). However, miR-30a overexpression was sufficient to durably improve serum glucose (Fig. 5B) and insulin profiles (Fig. 5C) for >30 days. Although enforced miR-30a expression in the subcutaneous WAT did not significantly impact body fat percentage (Fig. 5D), histological sections of the inguinal WAT pads indicated that miR-30a increased the number of adipocytes (Fig. 5E), which correlates with WAT hyperplasia and insulin sensitivity (3, 48). Serum adiponectin levels during the 1-mo-long experiment remained intact after ectopic miR-30a expression (Fig. 5F). Together, these results demonstrate miR-30a overexpression in subcutaneous WAT exerts long-lasting impacts on adipocyte expansion.
Fig. 5.
Durable effects of miR-30a transgenesis in obese mice. A: adenovirus-green fluorescent protein (Adv-GFP) or Adv-miR-30a was injected into the inguinal fat pad of diet-induced obese (DIO) mice fed high-fat diet for 14 wk. B: temporal glucose levels during the entire 35-day experiment. C: homeostatic model assessment for insulin resistance (HOMA-IR) index in mice fasted for 4 h (n = 4 mice/group). HOMA-IR index was analyzed on day 0 vs. day 30. D: total fat mass changes of DIO mice ectopically expressing Adv-GFP or Adv-miR-30a in inguinal white adipose tissue (iWAT; n = 4 mice/group). E: histology and adipocyte size in iWAT of Adv-GFP or Adv-miR-30a DIO mice (n = 3 mice/group). The no. of adipocytes (per mm2) was tabulated across four ×20 fields of view per mouse fat depot. Scale bar, 50 μm. F: serum adiponectin changes during the experiment (n = 4). All data are expressed as means ± SE; *P < 0.05. Statistical significance in B was determined by 2-way ANOVA with multiple comparisons and the Holm-Sidak method (α = 0.05). Statistical significance in C–F was determined by 2-tailed unpaired Student’s t test. Unless otherwise indicated, all end points were measured 35 days after the first injection.
The beige fat transcriptional program can be activated by miR-30a.
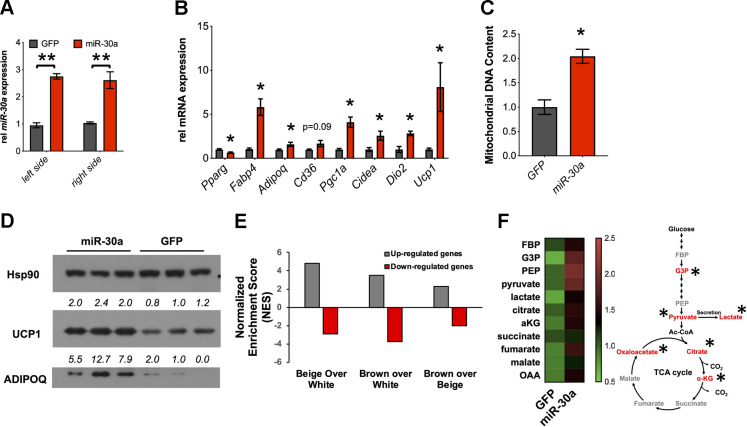
Our experiments suggest that miR-30a coordinates the beige fat program in vitro (Fig. 4) and enables lasting impacts on subcutaneous WAT hyperplasia in obese mice (Fig. 5). One month after injection, miR-30a levels were almost threefold higher in DIO mice ectopically expressing Adv-miR-30a in the two primary inguinal WAT depots compared with the control treatment groups (Fig. 6A). In agreement with our in vitro findings (35, 36), gene expression analysis showed increased expression of insulin sensitivity and mitochondrial lipid metabolism genes in miR-30a-infected WAT compared with the GFP control group (Fig. 6B). Ectopic miR-30a expression also induced mitochondrial DNA expansion (Fig. 6C). Consistent with beige fat gene expression and increased mitochondrial DNA, miR-30a expression in the inguinal WAT induced UCP1 protein levels (Fig. 6D).
Fig. 6.
Enforced miR-30a in subcutaneous white adipose tissue (WAT) promotes the beige fat transcriptional program. A and B: 35 days after the initial injection, quantitative PCR (qPCR) was used to measure expression of miR-30a (A) and genes associated with metabolic improvements (B) (n = 5; *P < 0.05, **P < 0.01). C: mitochondrial DNA (ND6) was analyzed by qPCR from samples in B; *P < 0.05 relative to green fluorescent protein (GFP) control. D: Western blotting with indicated antibodies to validate activation of the beige fat marker uncoupling protein 1 (UCP1). Heat shock protein 90 (HSP90) served as the invariant control. E: RNA-seq coupled with Gene Set Enrichment Analysis (GSEA) identified the overlap between genes affected by ectopic miR-30a expression in subcutaneous WAT of diet-induced obese (DIO) mice and clonally derived brown, beige, and white adipocytes. The normalized enrichment score (NES) reflects the degree to which a gene set is overrepresented at the top of the complete ranked gene list. F: targeted metabolomics analysis of subcutaneous WAT establishes adenovirus (Adv)-miR-30a and elevates levels of TCA cycle and glycolysis metabolites (n = 5 mice/group). a-KG, α-ketoglutarate; Ac-CoA, acetyl-CoA; G3P, glyceraldehyde 3-phosphate; GBP/FBP, glucose/fructose-1,6-bisphosphate; PEP, phosphoenolpyruvate. Red text reflects altered metabolites; *P < 0.05. Statistical significance in A was determined by 2-way ANOVA and Tukey post hoc tests. Statistical significance in B, C, and F was determined by 2-tailed unpaired Student’s t tests.
We leveraged RNA-seq data (GSE39342) to explore the transcriptome response after expressing miR-30a in the inguinal WAT of obese mice for 1 mo. Of the 705 total differentially expressed genes, 129 were upregulated (by ≥1.5–fold) in WAT injected with miR-30a. We then performed Gene Set Enrichment Analysis (GSEA) to interrogate the gene signature of ectopic miR-30a expression in subcutaneous WAT using a reference gene expression data set (accession no. GSE39562), which includes microarray data sets from clonally derived, differentiated brown and beige adipocyte cell lines (62). Notably, we found that genes affected by miR-30a overexpression were significantly enriched in beige and brown cells over white adipocytes (Fig. 6E).
Recent studies suggest that adipocytes adapt primary metabolic cycles to accommodate energetic demands and acquisition of beige fat cell features (29, 42). To investigate how miR-30a converts global gene expression changes into carbon metabolism, we performed targeted metabolomics analysis of inguinal WAT (Fig. 6F). Enforced miR-30a expression in inguinal WAT of obese mice did not broadly alter the levels of early glycolytic intermediates but caused accumulation of pyruvate and lactate. Downstream, some TCA cycle intermediates also accumulated, suggesting that miR-30a expression in the inguinal WAT impacts the cellular carbon pools for glycolysis and the TCA cycle. Collectively, ectopic miR-30a expression in the inguinal WAT of obese mice caused accrual of metabolites that form decision points for lactate production (pyruvate) and lipid synthesis (citrate). These data reinforce the notion that miR-30a expression in subcutaneous WAT of DIO mice is associated with beige adipocyte recruitment and reprogrammed metabolism.
DISCUSSION
In this study, we have found that miR-30a overexpression in human subcutaneous adipocytes resembles the beneficial effects of TZDs. We provide two primary lines of evidence that miR-30a coordinates expression of the metabolic programs for human adipocyte function. First, the enforced expression of miR-30a promoted TZD activity in human adipocytes. Second, we show that reduced miR-30a expression corresponds with similar depletion of mature adipocyte markers and insulin sensitivity in WAT isolated from diabetic patients. The latter observation suggests our in vitro findings establish human relevance. Our experiments also demonstrate miR-30a can exert therapeutic effects that resemble TZD actions, including subcutaneous WAT hyperplasia, beige adipocyte recruitment, and improved measures of insulin sensitivity in obese mice.
miR-30a exhibits broad tissue expression, which implies a vast target gene spectrum. A liberal survey of miR-30a targets identifies more than 2,400 targets, but target miRNA-mRNA interactions require tissue-specific functions and other biochemical contexts. To investigate direct connections between miR-30a and its target genes, we used our compendium of miRNA-target binding inferred by high-throughput sequencing (21, 22) and target prediction algorithms (1, 37). Among pathways that broadly oppose a differentiated state, miR-30a targets expression of critical cancer and motility genes, including KRAS, PIK3CD, EGFR, MET, and PDGFRB (38, 49). These genes coordinate the growth and migration of cancer cells in response to diverse autocrine and paracrine signals in the tumor microenvironment. Other studies support a role for miR-30a in human adipocytes (64), but further studies are required to identify the full spectrum of miR-30a targets and define how temporal miRNA expression directs adipocyte commitment and maintenance. Nonetheless, the abundance and selectivity of miR-30a for proliferative genes suggests a distinct function to maintain the mature adipocyte pool.
The −2-kb proximal promoter region of miR-30a harbors multiple transcription factor binding sites that likely allow functional regulation in adipocytes. In addition to PPARγ, motif analysis nominates sequences recognized by the glucocorticoid receptor (GR), C/EBP, and PPARα. Among PPAR isoforms expressed in adipose tissue, PPARγ expression remains the singular nuclear receptor capable of driving mature adipocyte functions (8), and adipose-specific activation of PPARγ reverses whole body insulin resistance to a degree similar to systemic TZD treatment (57). Therefore, although PPARα and PPARγ share similar DNA binding motifs, the tissue-specific expression patterns likely confer metabolic actions and selective regulation of miR-30a by PPARγ (17). Also, the location of GR-, C/EBP-, and PPARγ-binding sites near the miR-30a gene comprises an adipogenic hotspot (56). These transcription factors establish the primary regulon that generates and maintains the adipocyte phenotype. Because of the prolonged stability of mature miRNAs (4, 43), dynamic effects on transcriptional regulation are difficult to assess. However, the presence of functional and tissue-specific adipocyte transcription factor-binding sites suggests miR-30a regulation in response to changing hormonal and environmental cues. In future studies, we intend to identify which transcription factor(s) is essential for regulating the primary miR-30a gene in adipocytes.
TZDs improve insulin sensitivity by upregulating the expression of adipokine and lipid storage genes in WAT, many of which have been shown to be direct PPARγ target genes. Interestingly, TZD treatment also represses genes in vitro and in vivo, including the PPARγ gene itself (6, 20). In our studies, we establish that miR-30a can augment TZD activity and promote the acquisition of beige adipocyte features in primary human cells. To expand these studies, we manipulated miR-30a expression in the subcutaneous (inguinal) WAT of obese mice. We chose the inguinal fat pad for injection because no invasive surgical intervention is required. More importantly, mouse inguinal WAT is capable of inducible beige fat activity reflected by metabolic changes that durably reduced serum glucose levels and improve insulin sensitivity (30). At the morphological and gene expression levels, long-term miR-30a expression resembles TZD activity, including effects on adipocyte hyperplasia and gene expression (32).
Moreover, by integrating RNA-seq and GSEA, we established that miR-30a stimulates the beige and brown fat transcriptional program. Although miR-30a and TZD (55) broadly drive the beige fat transcriptional program, the cumulative effects share ∼10% of differentially impacted genes. Key genes in the overlap include ELOVL2, ELOVL3, CES1, and UCP1. One would suspect a more significant overlap of altered genes between miR-30a and TZD effects. Nonetheless, our findings suggest miR-30a and TZD effects exert similar effects on the beige adipocyte gene expression program.
Obesity frequently correlates with reduced mitochondrial function in adipose tissues. For example, expression of mitochondrial oxidative phosphorylation, glycolysis, and TCA cycle genes in human and mouse subcutaneous WAT are compromised under obese and diabetic states (11, 50, 51, 63). To this end, the metabolic benefits of TZDs partly derive from their ability to increase mitochondrial functions in adipocytes (7, 45). miR-30a supports regulation of the mitochondrial complement necessary to accelerate lipid and glucose metabolism in fat cells. In this setting, our metabolomics and RNA-seq analysis suggest that miR-30a likely spurs glycolysis, TCA cycle metabolism, and the mitochondrial electron transport chain to provide the metabolic intermediates for adipocyte hyperplasia in obesity. Moreover, the accumulation of citrate and pyruvate reflect greater carbohydrate metabolism and the accrual of lipogenic intermediates for fat cell expansion. Our data build on previous findings that the glucose sink function of beige fat regulates glucose clearance independently of body weight loss (13, 29, 52).
Identifying factors that enable adipocyte hyperplasia is fundamentally essential to understanding the expansion of adipose tissue in obesity. We analyzed data derived from high-throughput genome-wide methods (21, 22) to understand miRNA expression across tissues and guide interpretations of experiments performed in primary human adipocyte cells. Our investigations of miR-30a function leverage the most robust human in vitro models to demonstrate the durable correlation of miR-30a expression with measures of glucose and fatty acid metabolism in adipocytes. Our new data build upon recent findings (35, 36) and show that miR-30a supports beige adipocyte recruitment. Furthermore, emerging evidence suggests that increased beige fat biogenesis improves metabolic health in ways far beyond the induction of thermogenesis. For instance, selective beige fat recruitment improves systemic glucose tolerance and insulin sensitivity, reduces WAT inflammation and fibrosis, and protects against hepatic steatosis (13, 28, 29, 39). Along these lines, it will now be important to understand how miR-30a expression allows WAT expansion and protection from the comorbidities of obesity.
GRANTS
This work was funded by American Diabetes Association Grant no. 1-18-IBS-105 and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-114356. This study was also funded (in part) by an award from the Baylor College of Medicine Nutrition and Obesity Pilot and Feasibility Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.K.S. and S.M.H. conceived and designed research; P.K.S., M.P.H., K.R., V.P., M.B., N.P., C.C., and S.M.H. performed experiments; P.K.S., M.P.H., K.R., V.P., J.B.F., P.M., A.R.C., M.B., N.P., C.C., and S.M.H. analyzed data; P.K.S., M.P.H., K.R., V.P., J.B.F., A.R.C., M.B., N.P., C.C., and S.M.H. interpreted results of experiments; M.P.H., K.R., V.P., J.B.F., P.M., A.R.C., M.B., C.C., and S.M.H. prepared figures; K.R., M.B., and S.M.H. drafted manuscript; P.K.S., M.P.H., K.R., V.P., J.B.F., P.M., A.R.C., M.B., N.P., C.C., and S.M.H. edited and revised manuscript; P.K.S., M.P.H., K.R., V.P., J.B.F., P.M., A.R.C., M.B., N.P., C.C., and S.M.H. approved final version of manuscript.
REFERENCES
- 1.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife 4: e05005, 2015. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allgood VE, Zhang Y, O’Malley BW, Weigel NL. Analysis of chicken progesterone receptor function and phosphorylation using an adenovirus-mediated procedure for high-efficiency DNA transfer. Biochemistry 36: 224–232, 1997. doi: 10.1021/bi961125c. [DOI] [PubMed] [Google Scholar]
- 3.Andersson DP, Eriksson Hogling D, Thorell A, Toft E, Qvisth V, Näslund E, Thörne A, Wirén M, Löfgren P, Hoffstedt J, Dahlman I, Mejhert N, Rydén M, Arner E, Arner P. Changes in subcutaneous fat cell volume and insulin sensitivity after weight loss. Diabetes Care 37: 1831–1836, 2014. doi: 10.2337/dc13-2395. [DOI] [PubMed] [Google Scholar]
- 4.Bail S, Swerdel M, Liu H, Jiao X, Goff LA, Hart RP, Kiledjian M. Differential regulation of microRNA stability. RNA 16: 1032–1039, 2010. doi: 10.1261/rna.1851510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP Metazoan microRNAs. Cell 173: 20–51, 2018. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger JP, Petro AE, Macnaul KL, Kelly LJ, Zhang BB, Richards K, Elbrecht A, Johnson BA, Zhou G, Doebber TW, Biswas C, Parikh M, Sharma N, Tanen MR, Thompson GM, Ventre J, Adams AD, Mosley R, Surwit RS, Moller DE. Distinct properties and advantages of a novel peroxisome proliferator-activated protein [gamma] selective modulator. Mol Endocrinol 17: 662–676, 2003. doi: 10.1210/me.2002-0217. [DOI] [PubMed] [Google Scholar]
- 7.Bogacka I, Ukropcova B, McNeil M, Gimble JM, Smith SR. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. J Clin Endocrinol Metab 90: 6650–6656, 2005. doi: 10.1210/jc.2005-1024. [DOI] [PubMed] [Google Scholar]
- 8.Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J, Evans RM, Spiegelman BM. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev 10: 974–984, 1996. doi: 10.1101/gad.10.8.974. [DOI] [PubMed] [Google Scholar]
- 9.Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y, Nettles KW, Griffin PR. Partial agonists activate PPARgamma using a helix 12 independent mechanism. Structure 15: 1258–1271, 2007. doi: 10.1016/j.str.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 460: 479–486, 2009. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49: 784–791, 2006. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 12.Civelek M, Hagopian R, Pan C, Che N, Yang WP, Kayne PS, Saleem NK, Cederberg H, Kuusisto J, Gargalovic PS, Kirchgessner TG, Laakso M, Lusis AJ. Genetic regulation of human adipose microRNA expression and its consequences for metabolic traits. Hum Mol Genet 22: 3023–3037, 2013. doi: 10.1093/hmg/ddt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156: 304–316, 2014. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corcoran DL, Pandit KV, Gordon B, Bhattacharjee A, Kaminski N, Benos PV. Features of mammalian microRNA promoters emerge from polymerase II chromatin immunoprecipitation data. PLoS One 4: e5279, 2009. doi: 10.1371/journal.pone.0005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeg MA, Buse JB, Goldberg RB, Kendall DM, Zagar AJ, Jacober SJ, Khan MA, Perez AT, Tan MH; GLAI Study Investigators . Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30: 2458–2464, 2007. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- 16.Ding H, Zheng S, Garcia-Ruiz D, Hou D, Wei Z, Liao Z, Li L, Zhang Y, Han X, Zen K, Zhang CY, Li J, Jiang X. Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16. Nat Commun 7: 11533, 2016. doi: 10.1038/ncomms11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest 127: 1202–1214, 2017. doi: 10.1172/JCI88894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galarraga M, Campión J, Muñoz-Barrutia A, Boqué N, Moreno H, Martínez JA, Milagro F, Ortiz-de-Solórzano C. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res 53: 2791–2796, 2012. doi: 10.1194/jlr.D023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, Tan MH, Khan MA, Perez AT, Jacober SJ; GLAI Study Investigators . A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28: 1547–1554, 2005. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 20.Haakonsson AK, Stahl Madsen M, Nielsen R, Sandelin A, Mandrup S. Acute genome-wide effects of rosiglitazone on PPARγ transcriptional networks in adipocytes. Mol Endocrinol 27: 1536–1549, 2013. doi: 10.1210/me.2013-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton MP, Rajapakshe K, Hartig SM, Reva B, McLellan MD, Kandoth C, Ding L, Zack TI, Gunaratne PH, Wheeler DA, Coarfa C, McGuire SE. Identification of a pan-cancer oncogenic microRNA superfamily anchored by a central core seed motif. Nat Commun 4: 2730, 2013. doi: 10.1038/ncomms3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton MP, Rajapakshe KI, Bader DA, Cerne JZ, Smith EA, Coarfa C, Hartig SM, McGuire SE. The landscape of microRNA targeting in prostate cancer defined by AGO-PAR-CLIP. Neoplasia 18: 356–370, 2016. doi: 10.1016/j.neo.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammarstedt A, Gogg S, Hedjazifar S, Nerstedt A, Smith U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol Rev 98: 1911–1941, 2018. doi: 10.1152/physrev.00034.2017. [DOI] [PubMed] [Google Scholar]
- 24.Hartig SM, Bader DA, Abadie KV, Motamed M, Hamilton MP, Long W, York B, Mueller M, Wagner M, Trauner M, Chan L, Bajaj M, Moore DD, Mancini MA, McGuire SE. Ubc9 impairs activation of the brown bat energy metabolism program in human white adipocytes. Mol Endocrinol 29: 1320–1333, 2015. doi: 10.1210/me.2015-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartig SM, Hamilton MP, Bader DA, McGuire SE. The miRNA interactome in metabolic homeostasis. Trends Endocrinol Metab 26: 733–745, 2015. doi: 10.1016/j.tem.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartig SM, He B, Long W, Buehrer BM, Mancini MA. Homeostatic levels of SRC-2 and SRC-3 promote early human adipogenesis. J Cell Biol 192: 55–67, 2011. doi: 10.1083/jcb.201004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartig SM, He B, Newberg JY, Ochsner SA, Loose DS, Lanz RB, McKenna NJ, Buehrer BM, McGuire SE, Marcelli M, Mancini MA. Feed-forward inhibition of androgen receptor activity by glucocorticoid action in human adipocytes. Chem Biol 19: 1126–1141, 2012. doi: 10.1016/j.chembiol.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, Hosono T, Maretich P, Yang Y, Ishigaki Y, Chi J, Cohen P, Koliwad SK, Kajimura S. Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab 27: 180–194.e6, 2018. doi: 10.1016/j.cmet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23: 1454–1465, 2017. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda K, Maretich P, Kajimura S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab 29: 191–200, 2018. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John E, Wienecke-Baldacchino A, Liivrand M, Heinäniemi M, Carlberg C, Sinkkonen L. Dataset integration identifies transcriptional regulation of microRNA genes by PPARγ in differentiating mouse 3T3-L1 adipocytes. Nucleic Acids Res 40: 4446–4460, 2012. doi: 10.1093/nar/gks025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly IE, Han TS, Walsh K, Lean ME. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22: 288–293, 1999. doi: 10.2337/diacare.22.2.288. [DOI] [PubMed] [Google Scholar]
- 33.Khan MA, St. Peter JV, Xue JL. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 25: 708–711, 2002. doi: 10.2337/diacare.25.4.708. [DOI] [PubMed] [Google Scholar]
- 34.King AB A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care 23: 557, 2000. doi: 10.2337/diacare.23.4.557b. [DOI] [PubMed] [Google Scholar]
- 35.Koh EH, Chen Y, Bader DA, Hamilton MP, He B, York B, Kajimura S, McGuire SE, Hartig SM. Mitochondrial activity in human white adipocytes is regulated by the Ubiquitin Carrier Protein 9/microRNA-30a axis. J Biol Chem 291: 24747–24755, 2016. doi: 10.1074/jbc.M116.749408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh EH, Chernis N, Saha PK, Xiao L, Bader DA, Zhu B, Rajapakshe K, Hamilton MP, Liu X, Perera D, Chen X, York B, Trauner M, Coarfa C, Bajaj M, Moore DD, Deng T, McGuire SE, Hartig SM. miR-30a remodels subcutaneous adipose tissue inflammation to improve insulin sensitivity in obesity. Diabetes 67: 2541–2553, 2018. doi: 10.2337/db17-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42: D92–D97, 2014. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao WT, Ye YP, Zhang NJ, Li TT, Wang SY, Cui YM, Qi L, Wu P, Jiao HL, Xie YJ, Zhang C, Wang JX, Ding YQ. MicroRNA-30b functions as a tumour suppressor in human colorectal cancer by targeting KRAS, PIK3CD and BCL2. J Pathol 232: 415–427, 2014. doi: 10.1002/path.4309. [DOI] [PubMed] [Google Scholar]
- 39.Lin JZ, Martagón AJ, Cimini SL, Gonzalez DD, Tinkey DW, Biter A, Baxter JD, Webb P, Gustafsson JA, Hartig SM, Phillips KJ. Pharmacological activation of thyroid hormone receptors elicits a functional conversion of white to brown fat. Cell Reports 13: 1528–1537, 2015. doi: 10.1016/j.celrep.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative epigenomic analysis of murine and human adipogenesis. Cell 143: 156–169, 2010. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, Yoneshiro T, Spinelli JB, Lu GZ, Kazak L, Banks AS, Haigis MC, Kajimura S, Murphy MP, Gygi SP, Clish CB, Chouchani ET. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560: 102–106, 2018. doi: 10.1038/s41586-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105: 10513–10518, 2008. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 395: 137–143, 1998. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 45.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15: 395–404, 2012. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157: 1279–1291, 2014. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rydén M, Andersson DP, Bergström IB, Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab 99: E1870–E1876, 2014. doi: 10.1210/jc.2014-1526. [DOI] [PubMed] [Google Scholar]
- 49.Saleh AD, Cheng H, Martin SE, Si H, Ormanoglu P, Carlson S, Clavijo PE, Yang X, Das R, Cornelius S, Couper J, Chepeha D, Danilova L, Harris TM, Prystowsky MB, Childs GJ, Smith RV, Robertson AG, Jones SJM, Cherniack AD, Kim SS, Rait A, Pirollo KF, Chang EH, Chen Z, Van Waes C. Integrated genomic and functional microRNA analysis identifies miR-30-5p as a tumor suppressor and potential therapeutic nanomedicine in head and neck cancer. Clin Cancer Res 25: 2860–2873, 2019. doi: 10.1158/1078-0432.CCR-18-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schöttl T, Kappler L, Fromme T, Klingenspor M. Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol Metab 4: 631–642, 2015. doi: 10.1016/j.molmet.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schöttl T, Pachl F, Giesbertz P, Daniel H, Kuster B, Fromme T, Klingenspor M. Proteomic and metabolite profiling reveals profound structural and metabolic reorganization of adipocyte mitochondria in obesity. Obesity (Silver Spring) 28: 590–600, 2020. doi: 10.1002/oby.22737. [DOI] [PubMed] [Google Scholar]
- 52.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105, 2011. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith U Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest 125: 1790–1792, 2015. doi: 10.1172/JCI81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab 20: 573–591, 2014. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soccio RE, Li Z, Chen ER, Foong YH, Benson KK, Dispirito JR, Mullican SE, Emmett MJ, Briggs ER, Peed LC, Dzeng RK, Medina CJ, Jolivert JF, Kissig M, Rajapurkar SR, Damle M, Lim HW, Won KJ, Seale P, Steger DJ, Lazar MA. Targeting PPARγ in the epigenome rescues genetic metabolic defects in mice. J Clin Invest 127: 1451–1462, 2017. doi: 10.1172/JCI91211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ Jr, Lazar MA. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev 24: 1035–1044, 2010. doi: 10.1101/gad.1907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, Barish GD, Hong SH, Castro GL, Yin YQ, Nelson MC, Hsiao G, Greaves DR, Downes M, Yu RT, Olefsky JM, Evans RM. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci USA 106: 22504–22509, 2009. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 93: 359–404, 2013. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 59.Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia 50: 151–157, 2007. doi: 10.1007/s00125-006-0496-9. [DOI] [PubMed] [Google Scholar]
- 60.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542: 450–455, 2017. [Erratum in Nature 545: 252, 2017.] doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 278: 33370–33376, 2003. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 99: E209–E216, 2014. doi: 10.1210/jc.2013-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaragosi LE, Wdziekonski B, Brigand KL, Villageois P, Mari B, Waldmann R, Dani C, Barbry P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol 12: R64, 2011. doi: 10.1186/gb-2011-12-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]